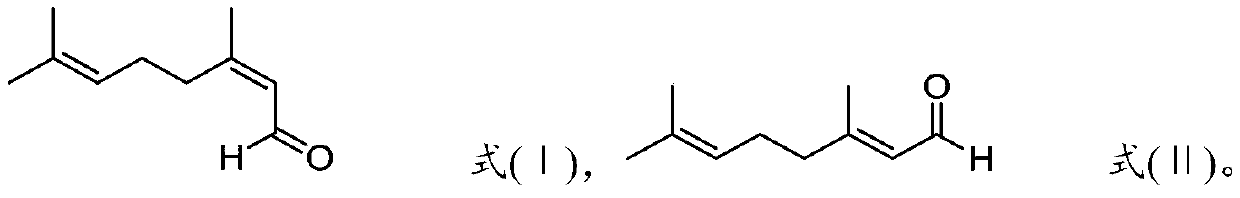
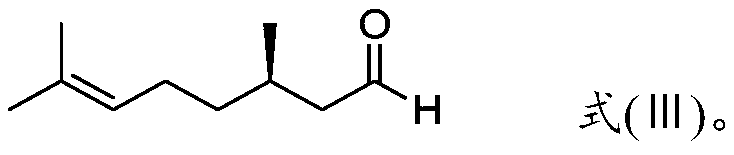
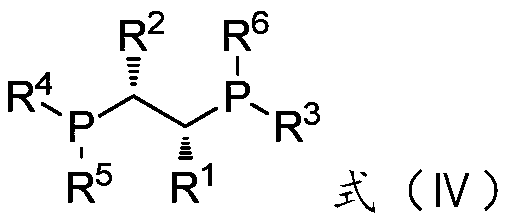
Preparation method of optically active citronellal
A technology for optically active and optically active ligands is applied in the field of preparing optically active citronellal, and can solve the problems of reduced catalyst conversion frequency, complex process operation, low hydrogenation efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1 (substrate raw material pretreatment)
[0066] The rectification column used for rectification and purification of neral and geranial aldehyde is composed of a vacuum jacketed rectification column with a length of 1 meter and an inner diameter of 50 mm. There is a triangular spiral random packing with a diameter of 1.5 mm inside. Decalin is used In the separation efficiency measurement of the mixture of cis-trans isomers (the molar ratio of cis-trans isomers is 1:1) under the top pressure of 1000Pa, the separation efficiency of the whole column was measured to be 41 theoretical plates. The tower is equipped with an oil-heated thin-film evaporator (the evaporation area is 0.07m 2 ) and a condenser cooled with cooling water.
[0067] Under the top pressure of 1000Pa, carry out batch rectification to 500g purity 99% neral, the heating temperature of the thin film evaporator at the bottom of the tower is 100 ℃, and the top condenser temperature is 20 ℃. After...
Embodiment 2
[0068] Embodiment 2 (substrate raw material pretreatment)
[0069] Use the rectifying tower described in embodiment 1, under the top pressure of 1000Pa, 500g purity is 99% geranial aldehyde is carried out intermittent rectification, the heating temperature of the thin-film evaporator at the bottom of the tower is 100 ℃, the top condenser temperature is 20°C. After total reflux for 1 hour to establish tower balance, adjust the reflux ratio to be 100:1 to carry out tower top extraction, switch to the geranial product collection tank after extracting 80g of the front fraction at the top of the tower, stop rectification after continuous extraction of 380g, and rectify to obtain The measured hydroxyl value of geranial is 1mgKOH / g, and the iron content is 60ppm.
Embodiment 3
[0070] Embodiment 3 (substrate raw material pretreatment)
[0071]Using the rectifying tower described in embodiment 1, under the top pressure of 1000Pa, 500g purity is carried out intermittent rectification to the neral aldehyde of 99%; is 20°C. After total reflux for 1 hour to establish tower balance, adjust the reflux ratio to be 150:1 to carry out tower top extraction, switch to the neral product collection tank after extracting 100g of the front fraction at the top of the tower, stop rectification after continuous extraction of 350g, and rectify to obtain The measured hydroxyl value of neral aldehyde is 1mgKOH / g, and the iron content is 60ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com