Method for preparing R-citronellal
A technology of citronellal and transition metals, applied in the field of R-citronellal preparation, can solve the problems of complex process operation, complex process, harsh recovery and application conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Add 8.9 grams of montmorillonite (montmorillonite K10) and 1 gram of nanosilver (20- 40 nanometers), after stirring at 30° C. for 1 hour, the montmorillonite and nano-silver were removed by filtration through a closed filter.
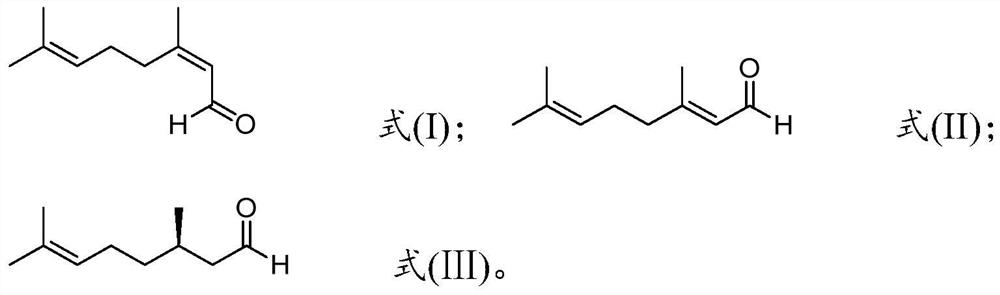
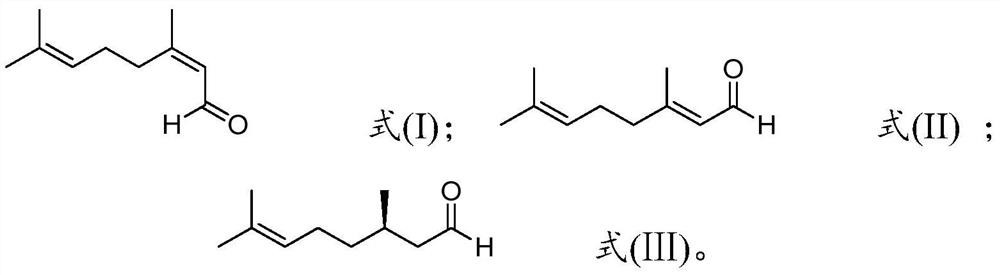
[0065] 7.5mg Rh(CO) 2 acac (0.029mmol) and 18.6mg (S,S)-chiraphos (0.436mmol) were dissolved in the above 8836g geranial (the molar ratio of geranial / neral double bond isomer=99:1); The molar calculation of rhodium atom in, substrate / catalyst molar ratio=2000000), join in the reactor, by injecting hydrogen, reaction pressure is adjusted to 80 bar (reaction pressure, bar), be warming up to 90 ℃ (reaction temperature) After reacting for 8 hours (reaction time), the conversion rate and optical purity measured by gas chromatography are listed in Table 1.
Embodiment 2-9
[0067] Examples 2-9 were carried out with reference to Example 1, except for the selection of aluminosilicate, nano-silver, reaction temperature, reaction pressure and reaction time, see Table 1 for details.
[0068] Table 1
[0069]
Embodiment 10
[0071] Add 1 gram of montmorillonite K10 to 8836g of neral aldehyde (the molar ratio of neral / geranial double bond isomers=99:1) under nitrogen atmosphere, stir at 10°C for 20 hours, pass The airtight filter removes the montmorillonite by filtration.
[0072] 7.5mg Rh(CO) 2 acac (0.029mmol) and 13mg (R, R)-chiraphos (0.030mmol) were dissolved in the above 8836g neral (the molar ratio of neral / geranial double bond isomer=99:1; Rhodium atom meter, substrate / catalyst molar ratio=2000000), was added in the reaction kettle, the reaction pressure was adjusted to 80 bar by injecting hydrogen, after the temperature was raised to 90°C for 8 hours, the conversion rate was measured by gas chromatography to be 53 %, the cumulative conversion number is 1,056,000, and the optical purity is 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com