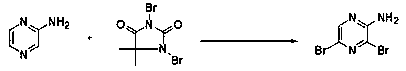
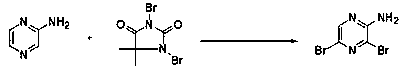
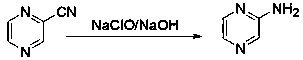Novel method for synthesizing 2-amino-3, 5-dibromopyrazine, product and application
A technology of dibromopyrazine and aminopyrazine, applied in the direction of organic chemistry, can solve the problems of large environmental pollution, unsuitable for industrial production, and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] Step 1. Preparation of 2-aminopyrazine: Add 10% sodium hypochlorite aqueous solution to the three-necked flask, cool down to below 0°C, add sodium hydroxide in batches, stir and dissolve until clear; -5°C~0°C Slowly add 2- Cyanopyrazine liquid, react for 5.8-6.2 hours after the dropwise addition; slowly drop the reaction solution into water (above 85°C), and react at 90~100°C until the end of the addition; evaporate the reaction solution under reduced pressure for 60 %~90% moisture, 15~20°C, stir for 0.8~1.5 hours, filter, and dry the filtered filter cake at 55~65°C; the dried filter cake is recrystallized with toluene, and dried to light yellow Crystalline 2-aminopyrazine, the reaction path is as follows:
[0053]
[0054] (I) (II)
[0055] Among them, the chemical formula of (I) is C 5 h 3 N 3 , molecular weight (Exact Mass): 105.03; (II) chemical formula is C 4 h 5 N 3 , molecular weight (Exact Mass): 95.05.
[0056] Step 2, mid-term reaction: Add N,N-dime...
Embodiment 1
[0077] Embodiment 1. A kind of new method of synthesizing 2-amino-3,5-dibromopyrazine
[0078] This example mainly describes the new method of synthesizing 2-amino-3,5-dibromopyrazine, specifically:
[0079] The preparation of step 1, 2-aminopyrazine
[0080] The operation process of the first step reaction is: add 105g of 10% sodium hypochlorite aqueous solution to a 3000ml three-necked bottle, cool down to below 0°C, add 125g of sodium hydroxide in batches, stir and dissolve until clear; -5°C~0°C slowly drop 2-cyanopyrazine liquid (the molar ratio of 2-cyanopyrazine and sodium hypochlorite is 1:1), react for 5.8~6.2 hours after the dropwise addition. Slowly add the reaction liquid dropwise into 105g (above 85°C) water, and react at 90~100°C until the end of the addition.
[0081] Distill 90% of the water in the reaction solution under reduced pressure, stir at 15-20°C for 1 hour, filter, and dry the filtered cake at 55-65°C; recrystallize the dried filter cake with 1.05kg ...
Embodiment 2
[0088] Embodiment 2. A kind of new method of synthesizing 2-amino-3,5-dibromopyrazine
[0089] This example mainly describes the new method of synthesizing 2-amino-3,5-dibromopyrazine, specifically:
[0090] The preparation of step 1, 2-aminopyrazine
[0091] The first step reaction operation process: Add 120g of 10% sodium hypochlorite aqueous solution to a 3000ml three-necked bottle, cool down to below 0°C, add 140g of sodium hydroxide in batches, stir and dissolve until clear; -5°C~0°C Slowly add 2- Cyanopyrazine liquid (the molar ratio of 2-cyanopyrazine and sodium hypochlorite is 1:1.2), react for 5.8~6.2 hours after the dropwise addition. Slowly add the reaction liquid dropwise into 105g (above 85°C) water, and react at 90~100°C until the end of the addition. Distill most of the water (60% of the water in the reaction solution) under reduced pressure, stir at 15~20°C for 1.5 hours, filter, and dry the filter cake at 55~65°C; recrystallize the solid with 1.05kg of tolue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com