Production method of potassium nitrate
A production method, the technology of potassium nitrate, applied in the chemical industry, can solve the problems of difficult separation of hydrochloric acid and nitric acid, no corrosion of aqua regia, and high corrosion of equipment, and achieve the effects of low cost, small corrosion of equipment, and large crystal particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of production method of potassium nitrate, comprises the following steps:
[0035] S1, adding nitric acid solution and magnesium oxide powder (purity: 99.5%) with a mass fraction of 42% in the reactor, reacted for 2.8 hours, then added potassium chloride, reacted for 2.5 hours, and obtained a reaction mixture;
[0036] The molar ratio of nitric acid, magnesium oxide and potassium chloride in the reactant is 1.8:1.4:1;
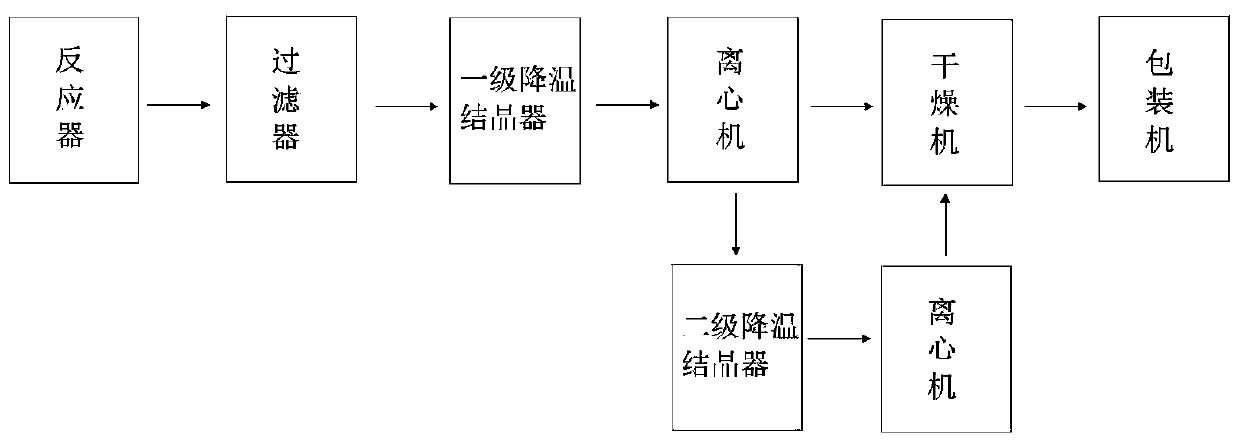
[0037] S2. Filter the reaction mixture obtained in S1 through a filter, remove impurities, and transport it to the first-stage cooling crystallizer for the first-stage cooling crystallization. The temperature is lowered to 35°C at a rate of 4.1°C / hour, and the potassium nitrate is obtained by centrifuge separation. Crystals and first-level mother liquor, the first-level mother liquor is transported to the second-level cooling crystallizer, and the second-level cooling crystallization is carried out, and the temperature is lowered to minus 12 °C at...
Embodiment 2
[0040] A kind of production method of potassium nitrate, comprises the following steps:
[0041] S1, add the nitric acid solution that the mass fraction is 50% and the magnesite of 91% magnesium oxide content in the reactor, after reacting for 2.2 hours, add potassium chloride again, react for 2.8 hours, obtain the reaction mixture;
[0042] The molar ratio of nitric acid, magnesium oxide and potassium chloride in the reactant is 1.6:1.3:1;
[0043] S2. Filter the reaction mixture obtained in S1 through a filter, remove impurities, and then transport it to a first-stage cooling crystallizer for first-stage cooling crystallization. The temperature is lowered to 31°C at a rate of 4.7°C / hour, and the potassium nitrate is obtained by centrifuge separation. Crystals and first-level mother liquor, the first-level mother liquor is transported to the second-level cooling crystallizer, and the second-level cooling crystallization is performed, and the temperature is lowered to minus 17...
Embodiment 3
[0046] A kind of production method of potassium nitrate, comprises the following steps:
[0047] S1, add the nitric acid solution that mass fraction is 33% and the magnesite of 94% magnesium oxide content in the reactor, react after 2.6 hours, add potassium chloride again, react for 1.8 hours, obtain reaction mixture;
[0048] The mol ratio of nitric acid, magnesium oxide, potassium chloride in the reactant is 1.7:1.2:1;
[0049] S2. Filter the reaction mixture obtained in S1 through a filter, remove impurities, and transport it to a first-stage cooling crystallizer for first-stage cooling crystallization. The temperature is lowered to 38°C at a rate of 3.8°C / hour, and the potassium nitrate is obtained by centrifuge separation. Crystals and first-level mother liquor, the first-level mother liquor is transported to the second-level cooling crystallizer, and the second-level cooling crystallization is performed, and the temperature is lowered to minus 15 °C at a rate of 5.2 °C / h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com