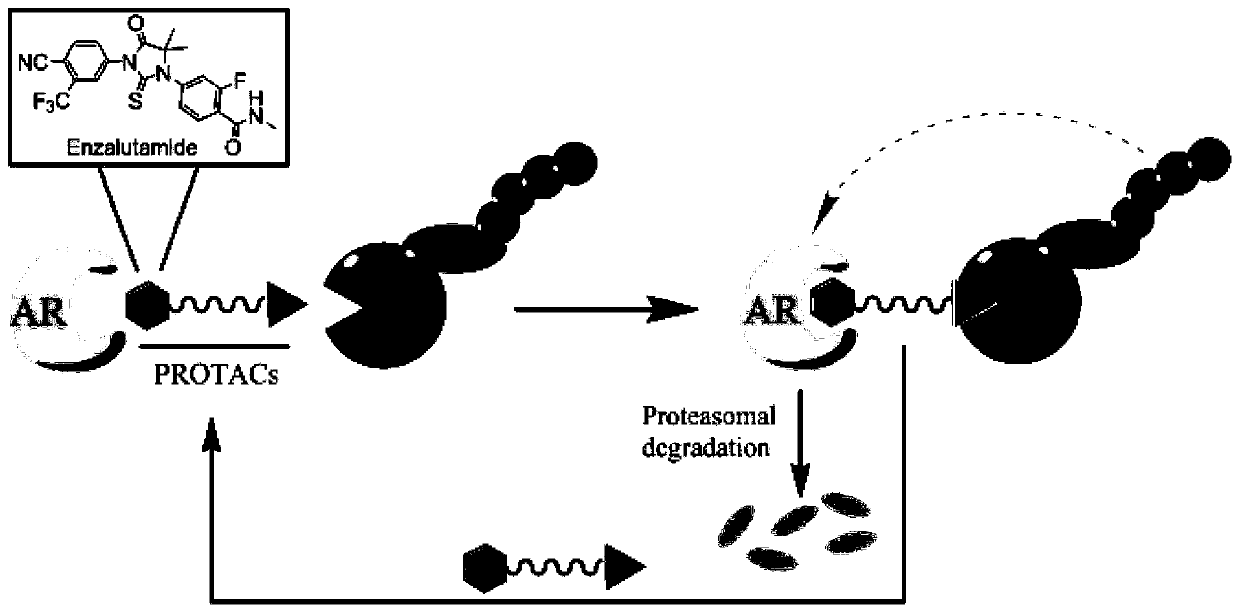
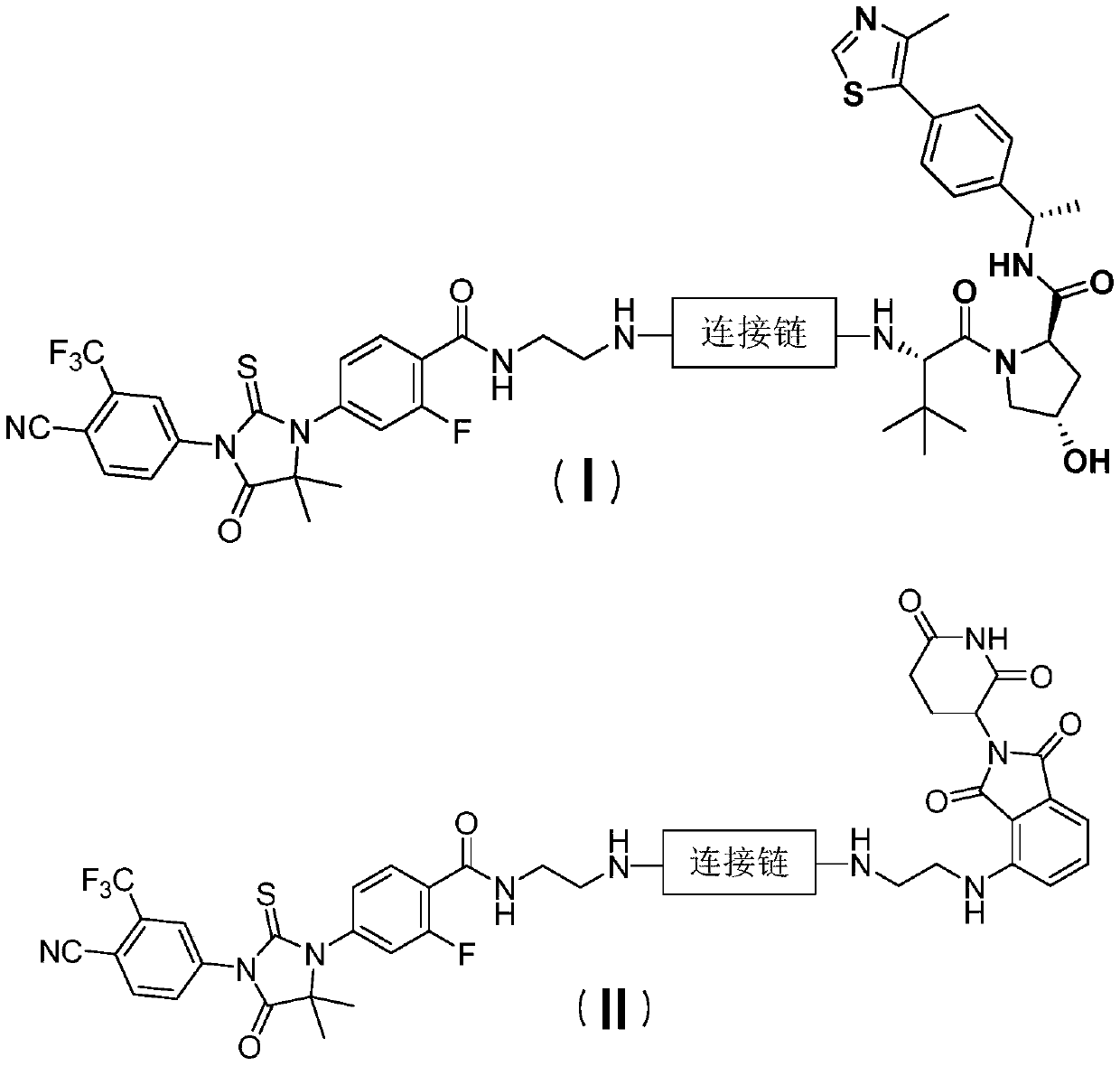
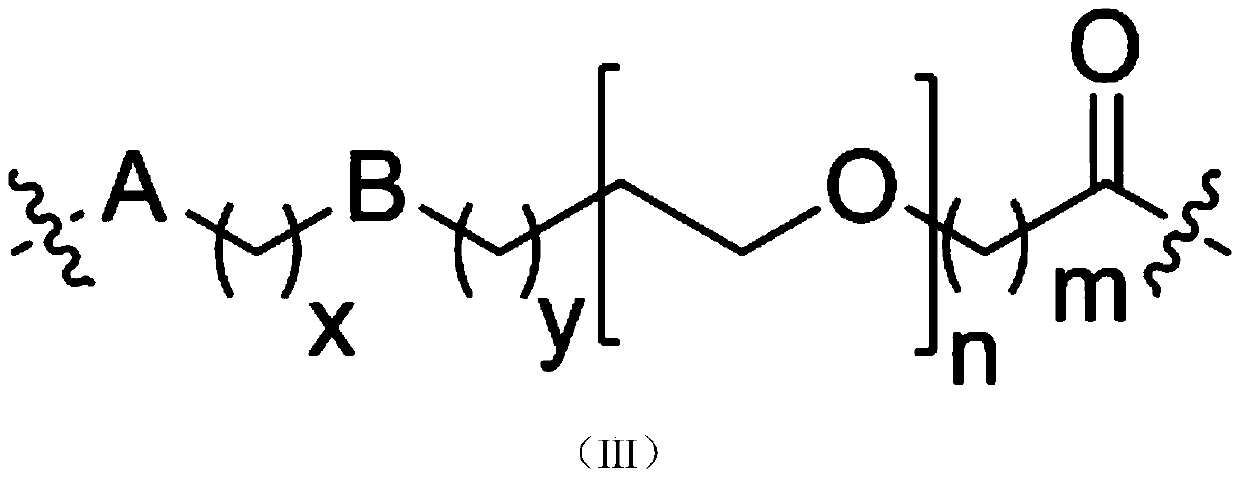
Small molecule degradation agent targeting at androgen receptors as well as preparation method and application thereof
A technology of androgen receptor and small molecules, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of intermediate Ⅰ-5
[0033]
[0034] The first step: preparation of intermediate Ⅰ-2
[0035] Add 2-fluoro-4-nitrotoluene I-1 (70g, 0.45mol) and tetrabutylammonium chloride (8.01g, 0.025mol) into a mixed solvent of 420mL water and 280mL tert-butanol, heat to reflux, After reflux, it was divided into five batches, slowly adding potassium permanganate (a total of 367.75g, 2.56mol) at intervals of 1.5h each time, reflux reaction for 30h, TLC detection (V 石油醚 :V 乙酸乙酯 =2:1) The reaction is complete, stand still until the temperature of the reaction solution is cooled to room temperature, filter with suction, add 6N HCl to the filtrate to adjust the pH to 1-2, extract with ethyl acetate, spin the ethyl acetate layer to obtain 71g of white solid, and collect rate of 85%. HRMS (ESI), calculated for C 7 h 4 FNO 4 186.0203[M+H] + , found 186.0236.
[0036] The second step: the preparation of intermediate Ⅰ-3
[0037] 1-Ethyl-3 (3-dimeth...
Embodiment 2
[0044] Embodiment 2: the synthesis of intermediate P-1
[0045]
[0046] The first step: the preparation of intermediate II-2
[0047] Add II-1 3-fluorophthalic anhydride (5g, 0.018mol), 3-amino-2,6-piperidinedione (2.31g, 0.018mol) and sodium acetate (1.78g, 0.022mol) into 60mL of acetic acid, Stir at room temperature to dissolve, heat up to 80°C, react at 80°C for 15h, TLC detection (V 二氯甲烷 :V 甲醇 =15:1) react completely, stop reaction, spin dry, polyamide column chromatography (V 二氯甲烷 :V 甲醇 =50:1) 6.7 g of golden yellow solid was obtained, with a yield of 81%. HRMS (ESI), calculated for C 13 h 9 FN 2 o 4 277.0625[M+H] + , found 277.0685.
[0048] The second step: the preparation of intermediate II-3
[0049] Add II-2 (3g, 0.011mol) into 30ml N,N-dimethylformamide, stir and dissolve at room temperature, and add N-tert-butoxycarbonylethylenediamine (2.61g, 0.016mol) and N , N-diisopropylethylamine (7.02g, 0.054mol), warming up to 90°C, reacting for 3h, TLC dete...
Embodiment 3
[0052] Embodiment 3: the synthesis of intermediate P-2
[0053]
[0054] The first step: synthesis of intermediate II-5
[0055] Add II-4 6-bromohexanoic acid (5 g, 0.026 mol) into 5 mL of thionyl chloride, stir at room temperature for 12 h, and spin dry to obtain an orange-yellow oil. HRMS (ESI), calculated for C 6 h 10 BrClO 212.9682[M+H] + , found 212.9661.
[0056] The second step: the synthesis of intermediate II-6
[0057] Add the oil obtained in the previous step into 30 mL of anhydrous N,N-dimethylformamide, stir and dissolve at room temperature, and add pomalidomide (3.50 g, 0.013 mol) after it is completely dissolved, N 2 Replace three times. Heat up to 105°C, react at 105°C for 3.5h, TLC detection (V 二氯甲烷 :V 甲醇 =20:1) The reaction is complete, stop the reaction, add 100mL of water to the reaction solution, extract with ethyl acetate, wash the ethyl acetate layer with saturated brine three times, dry over anhydrous sodium sulfate, spin dry to obtain 3.91g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com