PH response type curcumin and succinic anhydride prodrug nanometer micelle and preparation method and application thereof
A technology of curcumin succinic anhydride and nano micelles, which can be applied in nanotechnology, nanotechnology, nanotechnology and other directions for materials and surface science, and can solve the problems of poor absorption, low bioavailability, and poor water solubility of curcumin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] mPEG 350 -Synthesis of SA-Cur
[0079] (1) mPEG modified SA with a molecular weight of 350: Weigh 8g of mPEG respectively 350 (Polyethylene glycol monomethyl ether), 2.5 g of succinic anhydride, and 0.68 g of p-toluene sulfonic acid are placed in a flask under nitrogen environment protection and reacted at 80° C. for 3 hours. The product after the reaction was dissolved in 20ml of dichloromethane, filtered to take the filtrate, and then repeatedly recrystallized with 120ml of anhydrous ether at -15°C and freeze-dried to obtain the SA-modified mPEG product (mPEG 350 -SA);
[0080] (2)mPEG 350 -SA modified curcumin: weigh the mPEG product (mPEG) modified with SA described in step (1) 350 -SA) 1.8g, curcumin drugs (0.5g), N,N'-dicyclohexylcarbodiimide (1.2g), 4-dimethylaminopyridine (0.1g), triethylamine (0.1mL) added Ultra-dry dichloromethane solution (50ml), mix well, react for 12h under a nitrogen atmosphere, filter the reacted solution to remove the precipitate, then mix th...
Embodiment 2
[0083] mPEG 550 -Synthesis of SA-Cur
[0084] (1) mPEG modified SA with a molecular weight of 550: Weigh 8g of mPEG respectively 550 (Polyethylene glycol monomethyl ether), 1.6 g of succinic anhydride, and 0.56 g of p-toluene sulfonic acid are placed in a flask under nitrogen environment protection and reacted at 80° C. for 3 hours. The product after the reaction was dissolved in 30ml of dichloromethane, filtered to take the filtrate, and then repeatedly recrystallized with 160ml of anhydrous ether at -5°C and freeze-dried to obtain the SA-modified mPEG product (mPEG 550 -SA);
[0085] (2)mPEG 550 -SA modified curcumin: weigh the mPEG product (mPEG) modified with SA described in step (1) 550 -SA) 2.7g, curcumin drugs (0.54g), N,N'-dicyclohexylcarbodiimide (1.4g), 4-dimethylaminopyridine (0.1g), triethylamine (0.1mL) React in an ultra-dry dichloromethane solution (50ml) under a nitrogen atmosphere for 12h, filter the reacted solution to remove the precipitate, then mix the filtrate ...
Embodiment 3
[0088] mPEG 750 -Synthesis of SA-Cur
[0089] (1) mPEG modified SA with a molecular weight of 750: weigh 8g of mPEG respectively 750 (Polyethylene glycol monomethyl ether), 1.32g of succinic anhydride, 0.42g of p-toluenesulfonic acid in a flask, under nitrogen environment protection, react at 80°C for 3h. The reacted product was dissolved in 30ml of dichloromethane, filtered to take the filtrate, and then repeatedly recrystallized with 200ml of anhydrous ether at 0°C and freeze-dried to obtain the SA-modified mPEG product (mPEG 750 -SA);
[0090] (2)mPEG 750 -SA modified curcumin: weigh the mPEG product (mPEG) modified with SA described in step (1) 750 -SA) 2.5g, curcumin drugs (0.38g), N,N'-dicyclohexylcarbodiimide (0.9g), 4-dimethylaminopyridine (0.1g), triethylamine (0.1mL) In ultra-dry dichloromethane solution (50ml), mix well, react for 12h under a nitrogen atmosphere, filter the reacted solution to remove the precipitate, then mix the filtrate with 300mL of anhydrous ether an...
PUM
| Property | Measurement | Unit |
|---|---|---|
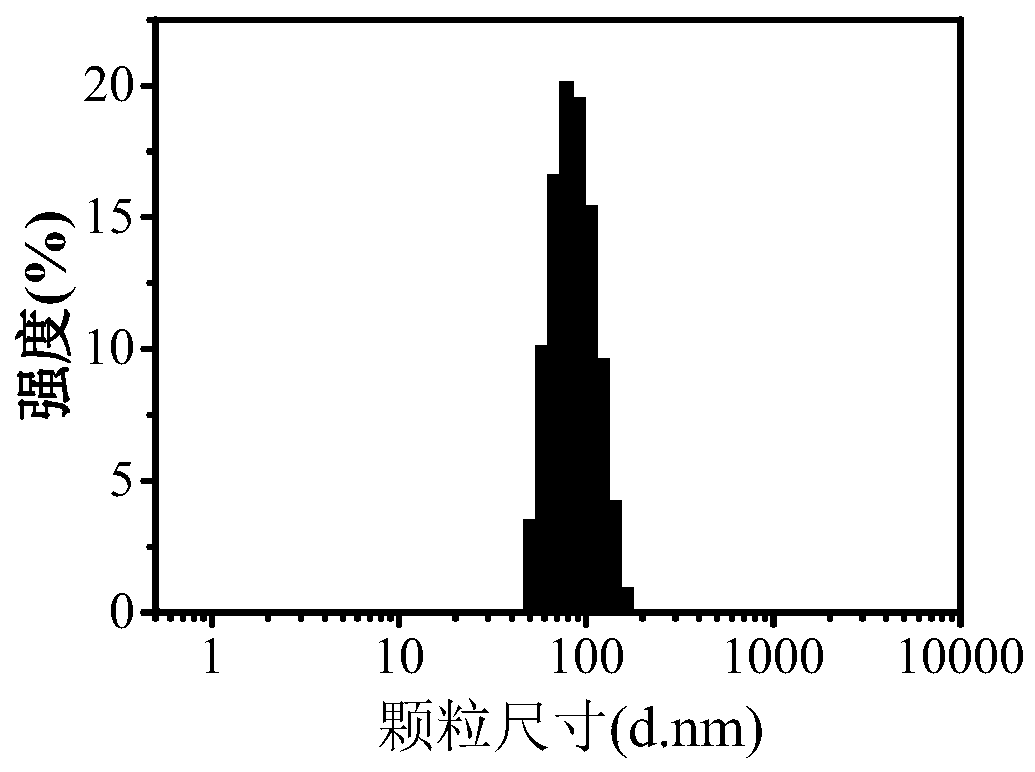
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com