Synthesis method of 5-nitroimidazole drugs
A technology of nitroimidazoles and nitroimidazoles, which is applied in the field of synthesis of 5-nitroimidazoles, can solve the problems of difficulty in obtaining sulfate esters, instability of sulfate esters, and low total yield, so as to reduce energy consumption and The effect of cost, reduction of three wastes, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a method for synthesizing 5-nitroimidazoles, comprising:
[0027] A) mixing 2-methyl-5-nitroimidazole, ethyl acetate and an aluminum chloride catalyst supported on a macroporous resin to obtain a mixed system;
[0028] B) Add epichlorohydrin or propylene oxide dropwise into the above mixed system and react to obtain 5-nitroimidazole antibacterial drugs.
[0029] The 5-nitroimidazole drug of the present invention is ornidazole or secnidazole. That is to say, the present invention discloses a synthetic method of ornidazole and a synthetic method of secnidazole.
[0030] The present invention mixes 2-methyl-5-nitroimidazole, ethyl acetate and an aluminum chloride catalyst supported on a macroporous resin to obtain a mixed system; 5-nitroimidazole, ethyl acetate and the aluminum trichloride catalyst supported on the macroporous resin are mixed.
[0031] The above mixing is preferably mixed in a reaction bottle, preferably a reactor with a stirring ...
Embodiment 1
[0057] Embodiment 1 (ornidazole)
[0058] In reaction bottle, add 330 g 2-methyl-5-nitroimidazoles, 1650 g ethyl acetate, add the aluminum chloride catalyst 1650 g that is loaded on the macroporous resin after opening stirring, stir well, stirring speed is 150 g rpm-200 rpm;
[0059] Add 330 g of epichlorohydrin dropwise to the reaction system at a rate of 3-5 ml / min, and control the internal temperature to not exceed 50 degrees;
[0060] After dripping epichlorohydrin, keep 45 degrees to react for 1.5 hours, filter, the catalyst is washed with 660 grams of ethyl acetate, filter again, and dry to be used mechanically; Pressure precipitation; add 330 grams of ethanol to the residue, heat to dissolve, cool down and crystallize, filter with suction, and dry to obtain the finished product of ornidazole.
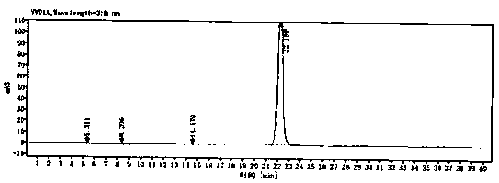
[0061] Detected by HPLC, the results are as follows: figure 1 said, figure 1 The HPLC figure of the ornidazole prepared for the embodiment of the present invention 1; By fig...
Embodiment 2
[0063] Embodiment 2 (secnidazole)
[0064] In reaction flask, add 330 g 2-methyl-5-nitroimidazoles, 1650 g ethyl acetate, add the aluminum chloride catalyst 1650 g that is loaded on the macroporous resin after starting to stir, stir well; Stir to be 150 rpm -200 rpm;
[0065] Add 300 g of propylene oxide dropwise to the reaction system at a rate of 3-5 ml / min, and control the internal temperature to not exceed 50 degrees;
[0066] After dropping the propylene oxide, keep it at 40°C for 1.5 hours, filter, wash the catalyst with 660 g of ethyl acetate, filter again, and dry it to be used mechanically; Precipitate; add 330 g of purified water to the residue, heat to dissolve, cool down and crystallize, filter with suction, and dry to obtain the finished product of ornidazole.
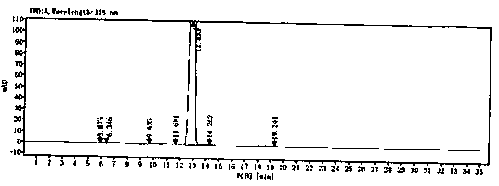
[0067] Detected by HPLC, the results are as follows: figure 2 said, figure 2 For the HPLC figure of the secnidazole prepared by the embodiment of the present invention 2, by figure 2 It can be calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com