Method for preparing epsilon-caprolactone through solvent-free cyclohexanone-benzaldehyde oxidation
A technology of cyclohexanone and benzaldehyde, which is applied in the field of ε-caprolactone by oxidation of ansolvated cyclohexanone-benzaldehyde, can solve the problems of high price, low reaction conversion rate, and inability to take advantage, and achieve a reduction in the process Energy consumption, the effect of reducing process energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0022] Solvent system: 1.306g of o-dichlorobenzene (internal standard), 0.4907g of cyclohexanone, 2.1224g of benzaldehyde, 100mg of nitrogen-doped carbon nanotubes and 17.5ml of the solvent in Table 1 were added to the autoclave.
[0023] Solvent-free system: Add 1.306g o-dichlorobenzene (internal standard), 8.5732g cyclohexanone, 9.3201g benzaldehyde, and 100mg nitrogen-doped carbon nanotubes into the autoclave.
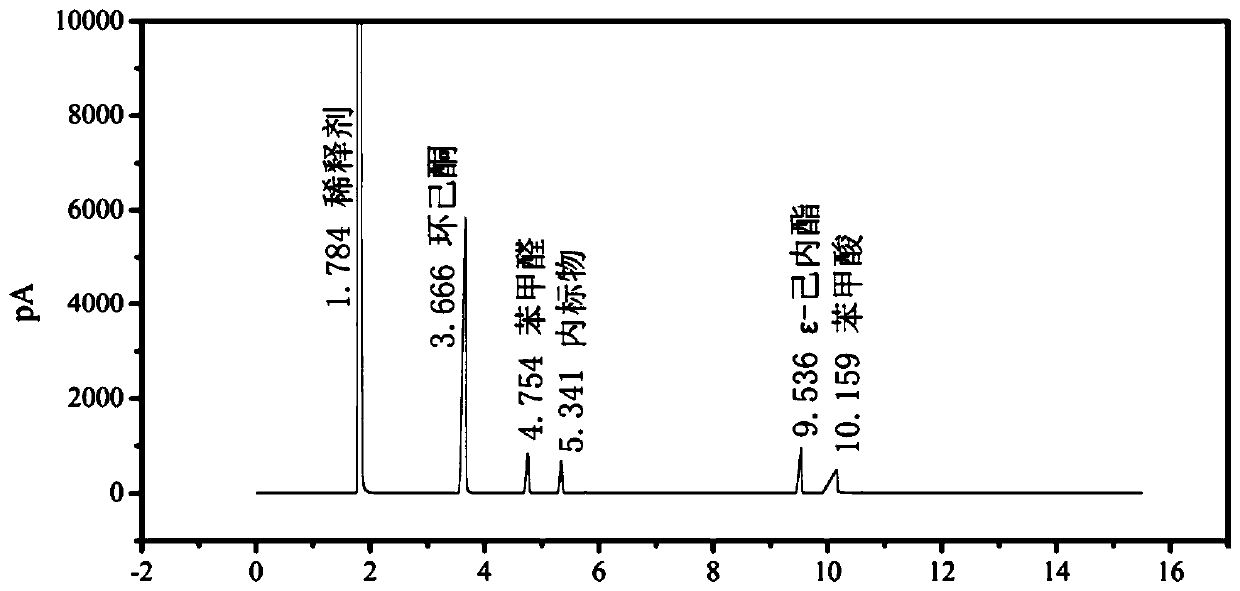
[0024] Both systems were stirred at a stirring rate of 1500rpm and heated to 85°C. Oxygen at a pressure of 1MPa was introduced, and timing was started. After 2 hours of reaction, the reaction vessel was cooled to 15°C in ice water, and the liquid-solid phase mixture was filtered to obtain a solid catalyst. And the liquid phase mixture containing unreacted reactant and reaction product, the liquid phase mixture is diluted 10 times with dichloroethane. GC detection results are shown in Table 1.
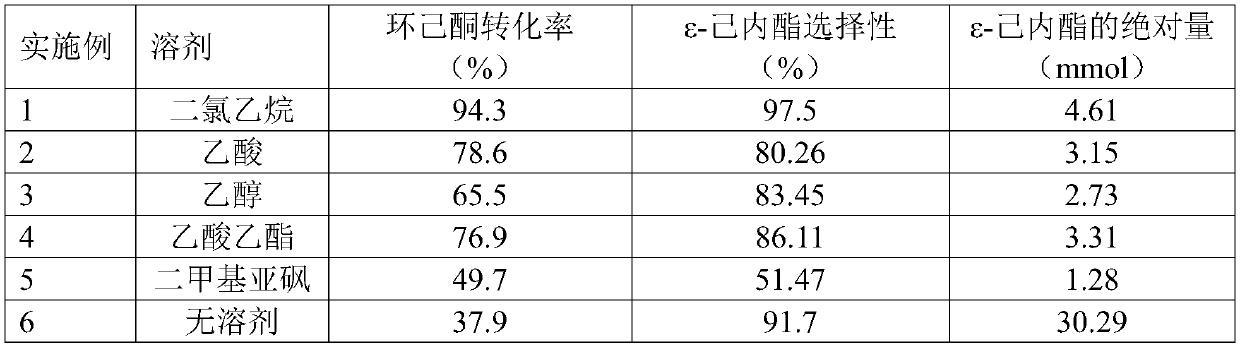
[0025] Table 1 has solvent B-V reaction performance contrast
[0026] ...
Embodiment 7
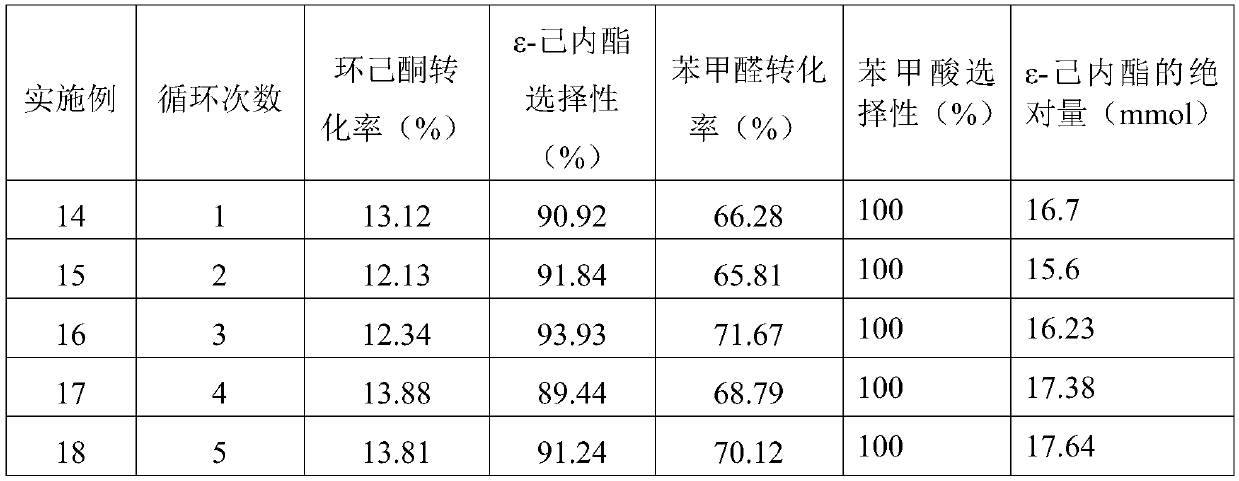
[0029] 1.306g of o-dichlorobenzene (internal standard), 13.68g of cyclohexanone, 14.976g of benzaldehyde and 100mg of nitrogen-doped carbon nanotubes with a nitrogen content of 5.25 at.% were added to the autoclave. Stir at a stirring rate of 1500 rpm and heat to 85° C., feed oxygen at a pressure of 1 MPa, and start timing. After reacting for 2 hours, cool the reactor to 15°C in ice water, filter the liquid-solid phase mixture to obtain a solid catalyst and a liquid phase mixture containing unreacted reactants and reaction products, and dilute the liquid phase mixture 10 times with dichloroethane . GC detection results: the conversion rate of cyclohexanone was 13.12%, the selectivity of ε-caprolactone was 91.4%, the conversion rate of benzaldehyde was 66.28%, the selectivity of benzoic acid was 100%, and the absolute production amount of ε-caprolactone was 16.7 mmol.
Embodiment 8
[0031] Add 1.306g o-dichlorobenzene (internal standard), 13.68g cyclohexanone, 14.976g benzaldehyde and 5mg nitrogen-doped carbon nanotubes (5.25at.% nitrogen content) into the autoclave and stir at a stirring rate of 2000rpm And heated to 85 ℃, through the oxygen pressure of 1MPa, start timing. After reacting for 2 hours, cool the reactor to 15°C in ice water, filter the liquid-solid phase mixture to obtain a solid catalyst and a liquid phase mixture containing unreacted reactants and reaction products, and dilute the liquid phase mixture 10 times with dichloroethane . GC detection results: the conversion rate of cyclohexanone is 15.22%, the selectivity of ε-caprolactone is 92.4%, the conversion rate of benzaldehyde is 69.28%, the selectivity of benzoic acid is 100%, and the absolute production amount of ε-caprolactone is 18.1 mmol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conversion rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com