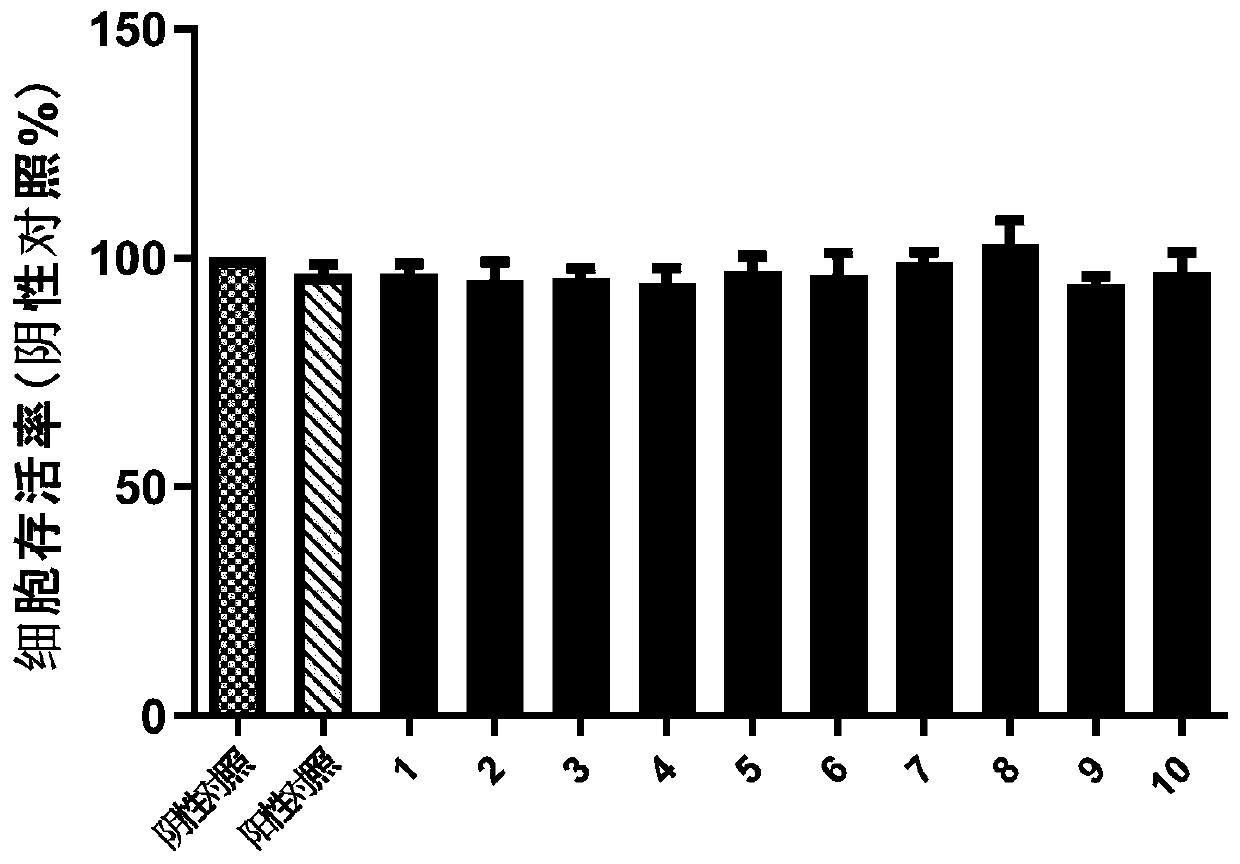
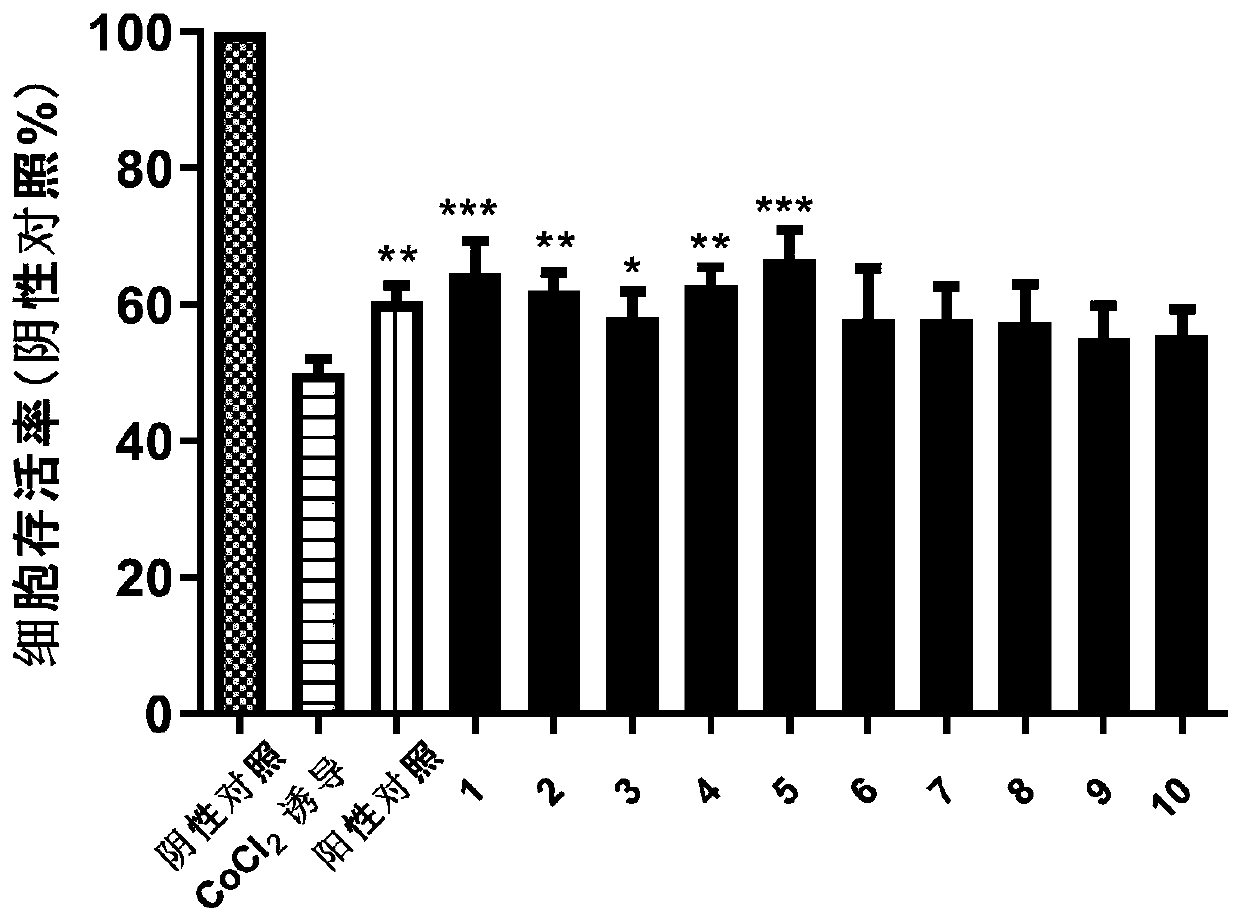
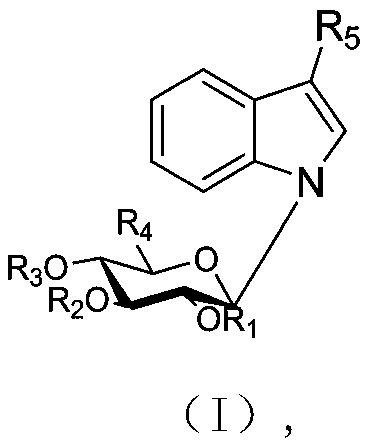
Indole N-glycoside compound, extraction method, and application in preparing drugs for preventing and treating nervous system diseases
A technology of indole azosides and compounds, which is applied in the field of indole azasides, which can solve the problems of no indole azapines and no PC12 cell damage protection, and achieve the effect of protecting cells from damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The extraction method of indole nitrogen glycoside compound comprises the following steps:
[0068] (1) Take 8.8 kg of dried and mature seeds of Sala chinensis, after coarse crushing, heat and reflux extraction with 10 mass times of 70% ethanol aqueous solution with a volume concentration of 3 times, each time for 3 hours, combine the extracts, and concentrate under reduced pressure to 65°C , to obtain 2.1kg of extract whose relative density is 1.20;
[0069] (2) Disperse the extract obtained in step (1) into distilled water of 5 times by mass, centrifuge to get the supernatant, carry out D101 type macroporous resin open column chromatographic separation (20 × 90cm), and use 4 times of column bed volume successively Water, 20%, 60%, and 95% ethanol aqueous solution were used for gradient elution, and the fractions eluted with 20% ethanol aqueous solution were collected, concentrated under reduced pressure to 65 ° C, and obtained 225 g of extract with a relative density ...
Embodiment 2
[0111] The extraction method of indole nitrogen glycoside compound comprises the following steps:
[0112] (1) Take 8.8 kg of dried and mature seeds of Sala chinensis, after coarse crushing, heat and reflux extraction with 8 mass times of 60% ethanol aqueous solution with a volume concentration of 4 times, each time for 3 hours, combine the extracts, and concentrate under reduced pressure to 65°C , to obtain an extract whose relative density is 1.15;
[0113] (2) the medicinal extract that step (1) is obtained is dispersed into the distilled water of 7 mass times, through HP 20 type macroporous adsorption resin column chromatography (20 * 90cm), with the water of 4 times column bed volume successively, volume concentration is Gradient elution with 20%, 60% and 95% ethanol aqueous solution, collect the fractions eluted with 20% ethanol aqueous solution, concentrate under reduced pressure to 65°C, and extract with a relative density of 1.25;
[0114] (3) Disperse the medicinal ...
Embodiment 3
[0117] The extraction method of indole nitrogen glycoside compound comprises the following steps:
[0118] (1) Take 8.8 kg of dried and mature seeds of Sala chinensis, after coarse crushing, heat and reflux extraction with 9 mass times of ethanol aqueous solution with a volume concentration of 65% for 2 times, each time for 3 hours, combine the extracts, and concentrate under reduced pressure to 65°C , to obtain an extract whose relative density is 1.25;
[0119] (2) Disperse the extract obtained in step (1) into distilled water of 10 mass times, through AB-8 type macroporous adsorption resin column chromatography (20 × 90cm), use 4 times of column bed volume water, volume concentration successively For gradient elution with 20%, 60% and 95% ethanol aqueous solution, collect the fractions eluted with 20% ethanol aqueous solution, concentrate under reduced pressure to 65°C, and extract with a relative density of 1.20;
[0120] (3) Disperse the extract obtained in step (2) into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com