Application of pheretima protein peptide in preparation of drugs for preventing and/or treating thrombotic diseases
A technology of ground dragon protein peptide and thrombotic disease, applied in the application field of medicine, can solve the problems of unsatisfactory treatment effect and single treatment effect, achieve good anticoagulant effect, anticoagulant fibrinolysis, and prevent and treat thrombosis effect of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The separation and purification of embodiment 1 earthworm protein peptide
[0045] The separation and purification steps of the earthworm protein peptide include the following steps:
[0046] 1) 10 g of earthworm peptide (provided by Zhongshi Duqing (Shandong) Biological Co., Ltd.), was added to 200 mL of 2% acetic acid aqueous solution and stirred for 0.5 h to dissolve. The ground dragon protein peptide was centrifuged at 8000r / min, 15°C for 15min, filtered through a filter head with a pore size of 0.45μm, and then used for later use.
[0047] 2) Separation of Sephadex G50 chromatographic column: First, wash the column with ultrapure water for 3 to 5 column volumes and load the sample, then wash the column with ultrapure water at 2.5mL / min for 2 hours, and the terrapin peptide after passing through the membrane is separated according to the chromatographic peak Fractions were collected and each fraction was concentrated and then freeze-dried in a vacuum dryer into a p...
Embodiment 2
[0056] 1) Determination of basic physical and chemical properties of earthworm protein peptide
[0057] Determination of protein content (GB5009.9-Kjeldahl nitrogen analyzer), determination of moisture content (GB5009.3), determination of ash content (GB5009.4), the measurement results are shown in Table 1.
[0058] Table 1 Determination results of basic physicochemical properties of earthworm protein peptide
[0059] protein moisture Ash 75.7% 5.29% 5.1%
[0060] 2) Analysis of amino acid distribution in earthworm protein peptide
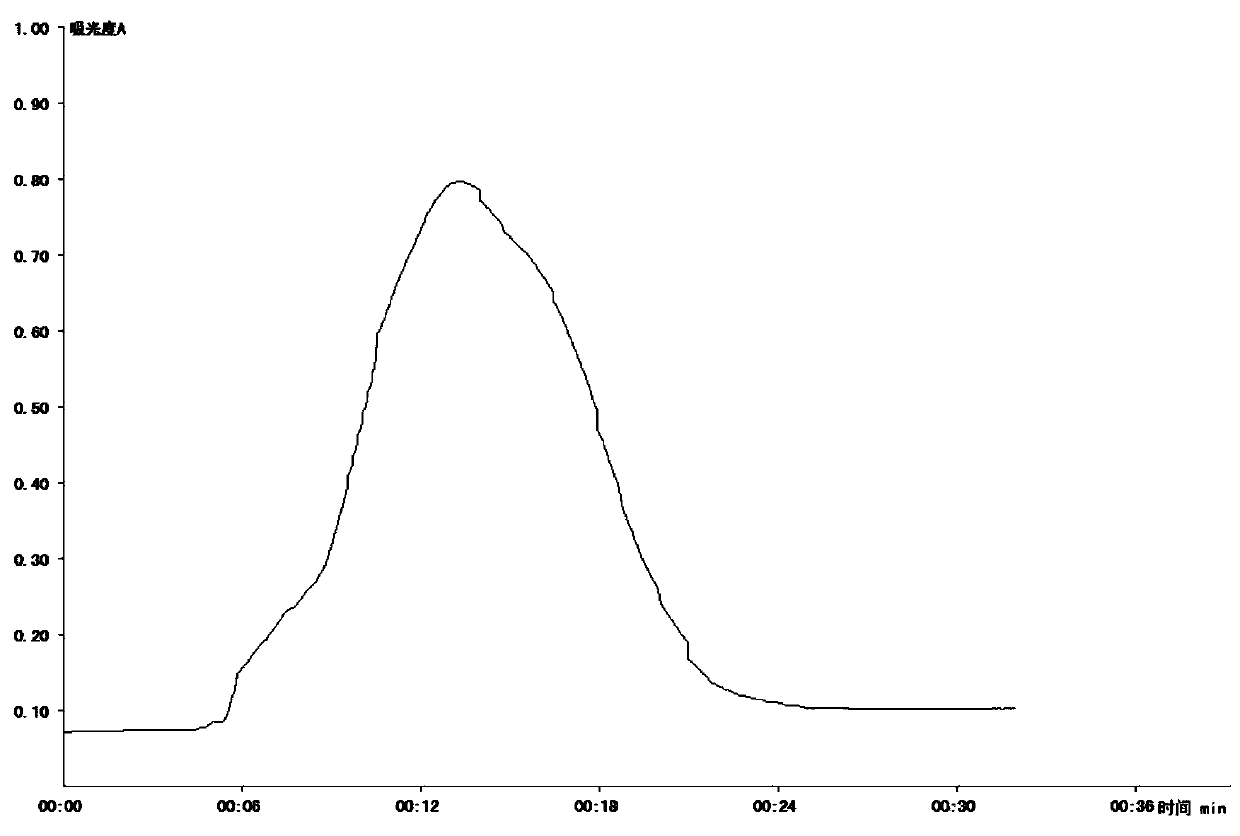
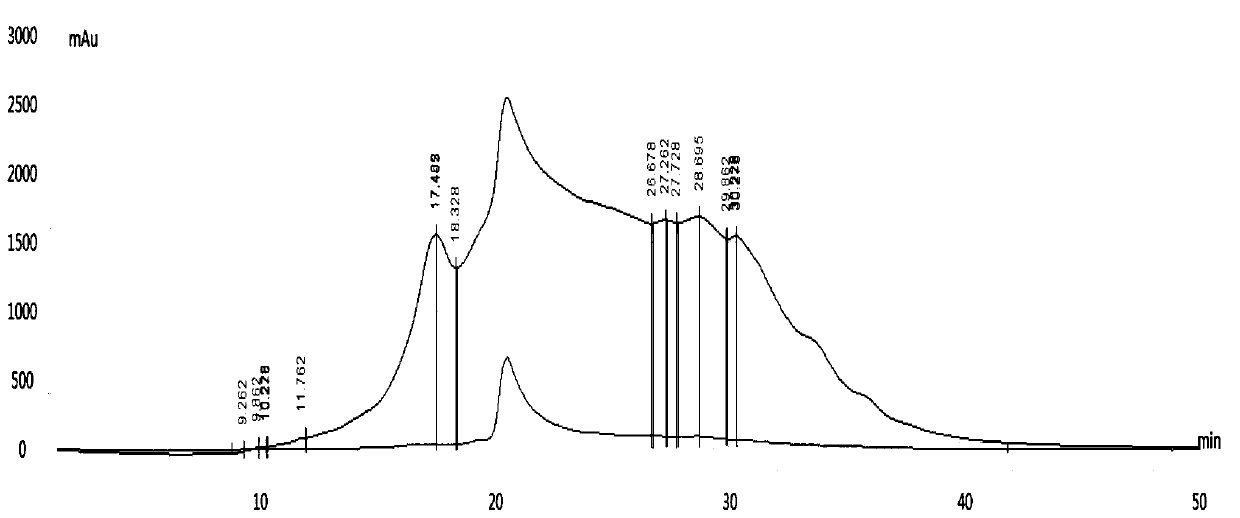
[0061] The amino acid composition of Dilong protein peptide was analyzed by Hitachi L-8900 amino acid automatic analyzer. The analysis results are detailed in Figure 7 . Depend on Figure 7 The distribution of the amino acid composition of the earthworm protein peptide can be known.
[0062] 3) Determination of molecular weight distribution of earthworm protein peptide
[0063] The molecular weight distribution of Di...
Embodiment 3
[0064] Example 3 Inhibitory Effect of Dilongin Peptide on Carrageenan-Induced Tail Thrombosis in Rats
[0065] 1) Reagents and instruments
[0066] Carrageenan was provided by Sagma; vernier caliper; stopwatch; scalpel, dilongin peptide, yellow powder, provided by Zhongshi Duqing (Shandong) Biological Co., Ltd., aspirin, white flake, batch number H51021360, BAIOO Lumbrokinase capsules, yellow capsules, batch number H11021129, lidocaine hydrochloride injection, injection, batch number H37022147.
[0067] 2) Modeling method
[0068] Take carrageenan, and be mixed with 0.9%NaCl concentration and be 2mg / mL carrageenan solution (2%); Give weight to rat, inject carrageenan subcutaneously in rat, dosage is 80mg / kg, The prevention group was given intragastric administration 5 days in advance, and 30 minutes after modeling, the other 4 groups except the prevention group were given intragastric administration according to the relevant dose, and then the rats were placed in a constant ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com