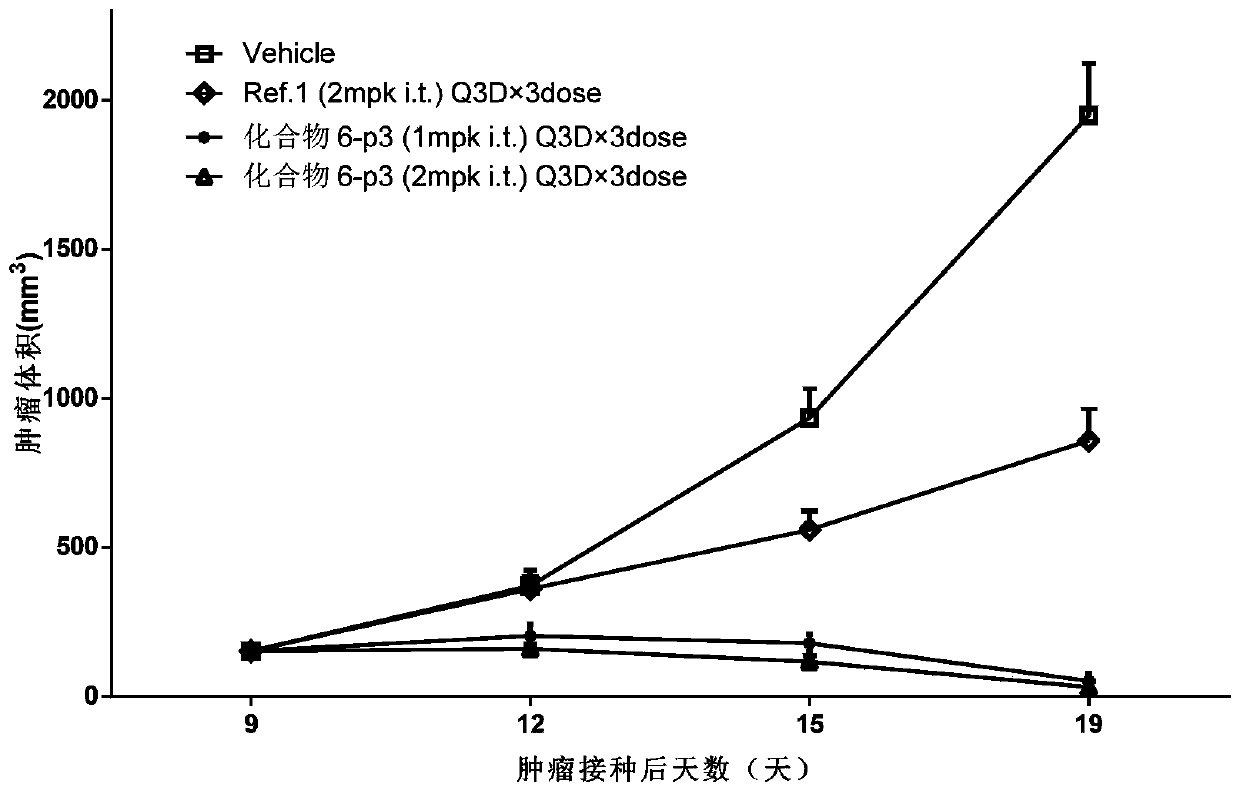
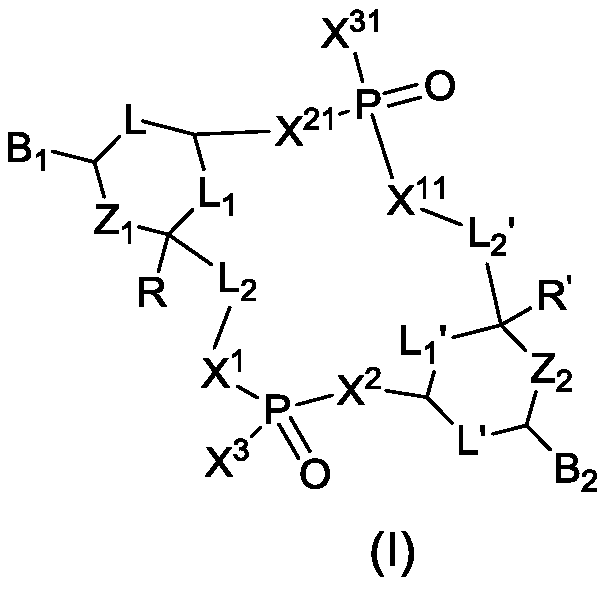
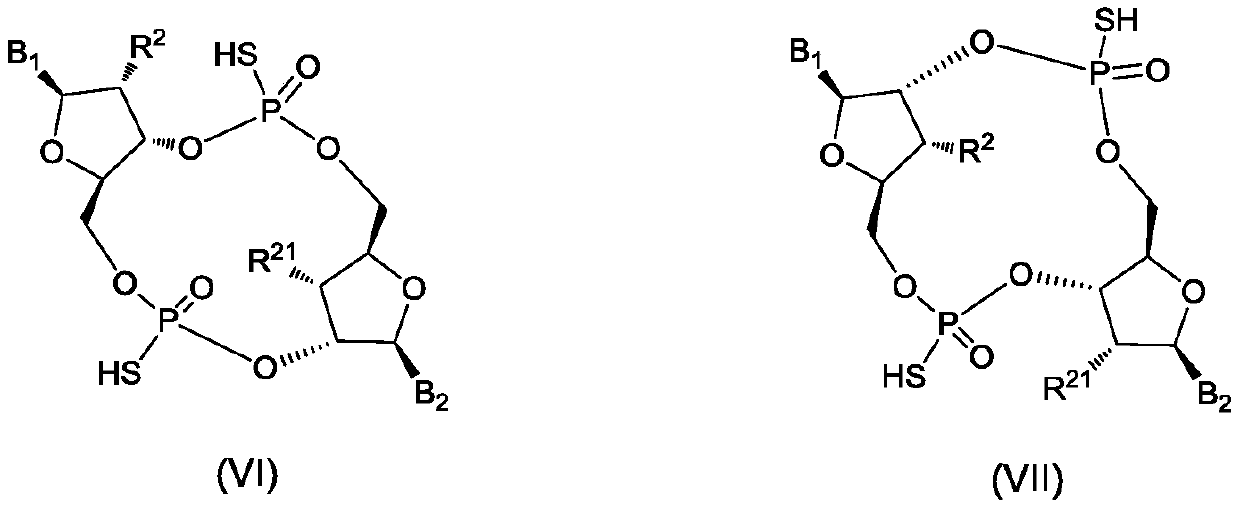
Cyclic dinucleotide analogues, pharmaceutical composition of analogues and applications of analogues and pharmaceutical composition
A technology of cyclic dinucleotides and analogues, applied in drug combinations, sugar derivatives, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0380] Embodiment 1: the synthesis of intermediate 1-8
[0381]
[0382]
[0383] Step 1: Suspend adenosine (50g, 187mmol) in acetic acid / sodium acetate (pH=4.0, 0.5M, 1L) buffer, slowly add liquid bromine (60g, 374mmol) dropwise, keep the system temperature below 10°C, After the addition was complete, the reaction system was stirred at room temperature for 48 hours. Add saturated aqueous sodium bisulfite solution to the reaction solution, remove excess bromine, adjust pH to neutral with aqueous sodium hydroxide solution (1M), cool in an ice-water bath, stir for 2 hours, precipitate solid, filter, collect solid, Intermediate 1-1 (29 g) was obtained after vacuum drying. m / z:[M+H] + 346.0 / 348.0.
[0384] Step 2: Intermediate 1-1 (10g, 28.9mmol) was suspended in methanol (100mL), sodium methoxide (9.36g, 173mmol) was added, the reaction system was refluxed and stirred for 5 hours, concentrated under reduced pressure to remove methanol, and the residue was dissolved in me...
Embodiment 2
[0390] Embodiment 2: the synthesis of compound 1-p1, 1-p2, 1-p3 and 1-p4
[0391]
[0392] Step 1: Intermediate 1-8 (2.85mmol) was dissolved in dry acetonitrile (15mL), concentrated under reduced pressure to remove the solvent, and the above operation was repeated twice. The last time left 10mL of acetonitrile, and No. 4 molecular sieves (0.8g) were added. Compound 1-9 (CAS No: 104992-55-4, 3.3g, 3.42mmol) was dissolved in dry acetonitrile (15mL), and then concentrated under reduced pressure to remove the solvent. After repeating twice, 5mL of acetonitrile remained for the last time. At 0°C, the acetonitrile solution of compound 1-9 was slowly added dropwise to the acetonitrile solution of compound 1-8, and the resulting reaction system was stirred at room temperature for 0.5 hours, and then N,N-dimethyl-N'- (3-Thio-3H-1,2,4-dithiazol-5-yl)formamidine (DDTT, 697mg, 3.42mmol) was added to the reaction liquid and stirred for 40 minutes. Molecular sieves were removed by filtr...
Embodiment 3
[0402] Example 3: Synthesis of Intermediates 2-8 and 2-9
[0403]
[0404] Step 1: Under nitrogen protection, add N,O-bistrimethylsilylacetamide (BSA, 13.6g, 116mmol) to 7-aminothiazolo[4,5-d]pyrimidin-2(3H)-one (synthetic The method refers to J.Med.Chem.1990, 33, 407-415 Compound 28) (6.5g, 38.7mmol) and tetraacetyl ribose (11g, 46.4mmol) in acetonitrile (120mL) solution, reflux for 1 hour. After the reaction solution was cooled to room temperature, trimethylsilyl trifluoromethanesulfonate (TMSOTf, 17.2 g, 77.4 mmol) was added to the above reaction solution, and reflux was continued for 48 hours. After the reaction solution was cooled to room temperature, slowly add saturated aqueous sodium bicarbonate solution to the above reaction solution, extract with ethyl acetate (150mL×3), combine the organic phases and wash with water, separate the organic phases and dry them with anhydrous sodium sulfate, filter, After concentration, the residue was purified by Flash column chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com