Preparation method for polyester with side chain containing dimethyl pyridylamine
A lutidine and side chain technology is applied in the field of preparation of polyesters containing lutidine in side chains, and can solve the problem that hydrophobic drugs and hydrophilic genes cannot be co-transported, and aliphatic polyesters are hydrophilic Insufficient sex and other problems to achieve good gene compounding ability and broaden the effect of biological applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
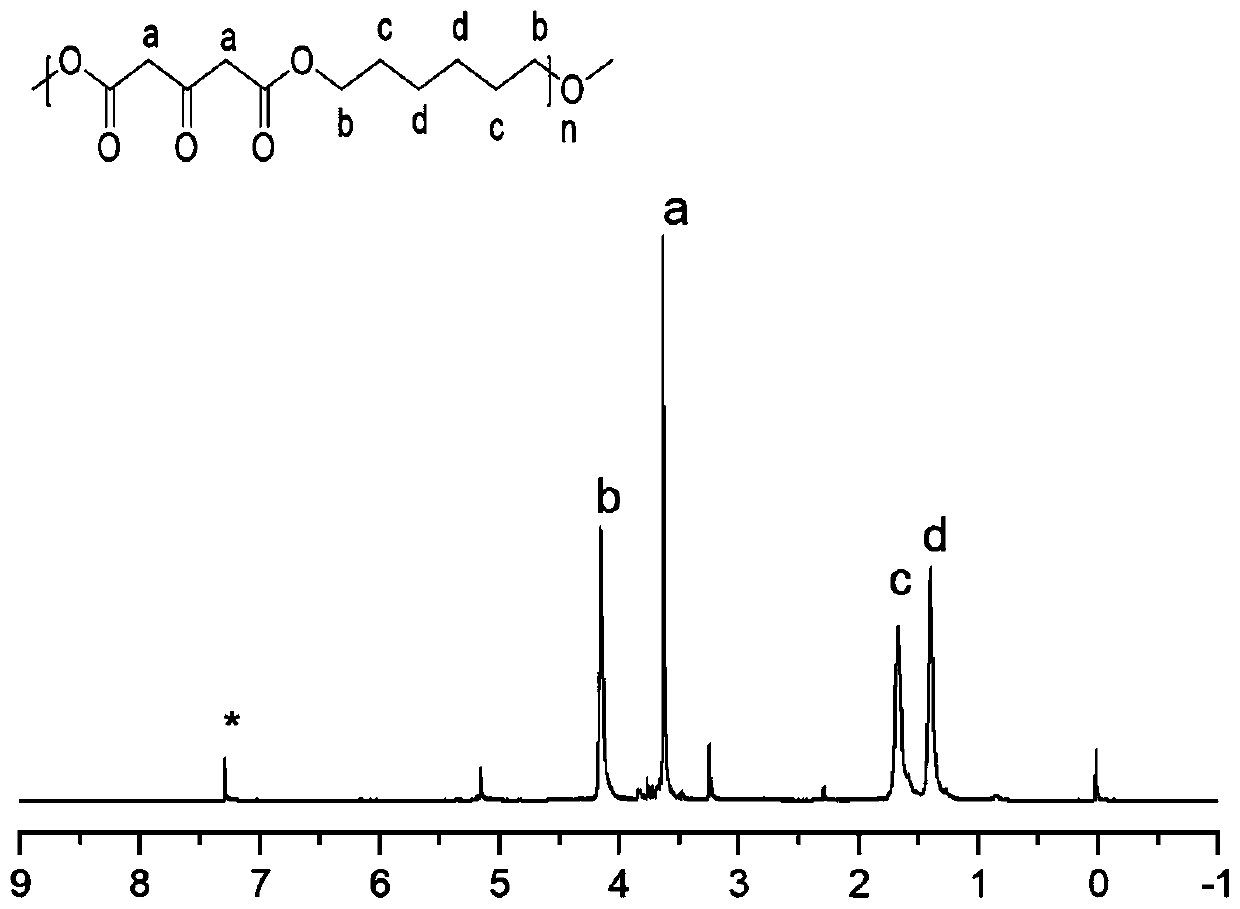
[0022] Taking the monomer B as 1,6-hexanediol as an example to calculate: use a precision balance to accurately weigh 3.588g (0.02mol, 1eq) dimethyl 1,3-acetonedicarboxylate, put it in a 20mL reaction bottle, and then accurately weigh Take 2.363g (0.02mol, 1eq) 1,6-hexanediol, and finally weigh 102mg (3*10 -4 mol, 0.015eq) TBT, and add magnetons, place in a 100°C round table, raise the temperature by 10°C every 20min, until the temperature rises to 150°C, and keep at 150°C for 4h. Stop the reaction, cool to room temperature, add 4-5ml of chloroform to heat and sonicate until dissolved. Add 15mL of methanol in a 20mL centrifuge tube, add 2mL of product each time, centrifuge at 6000r / min for 5min to remove remaining raw materials and oligomers, and the product obtained by centrifugation is dried in a vacuum oven for 24h. NMR characterization as figure 1 shown. Similar polymers can be obtained by expanding diol monomers into a series of aliphatic diols of different lengths, an...
Embodiment 2
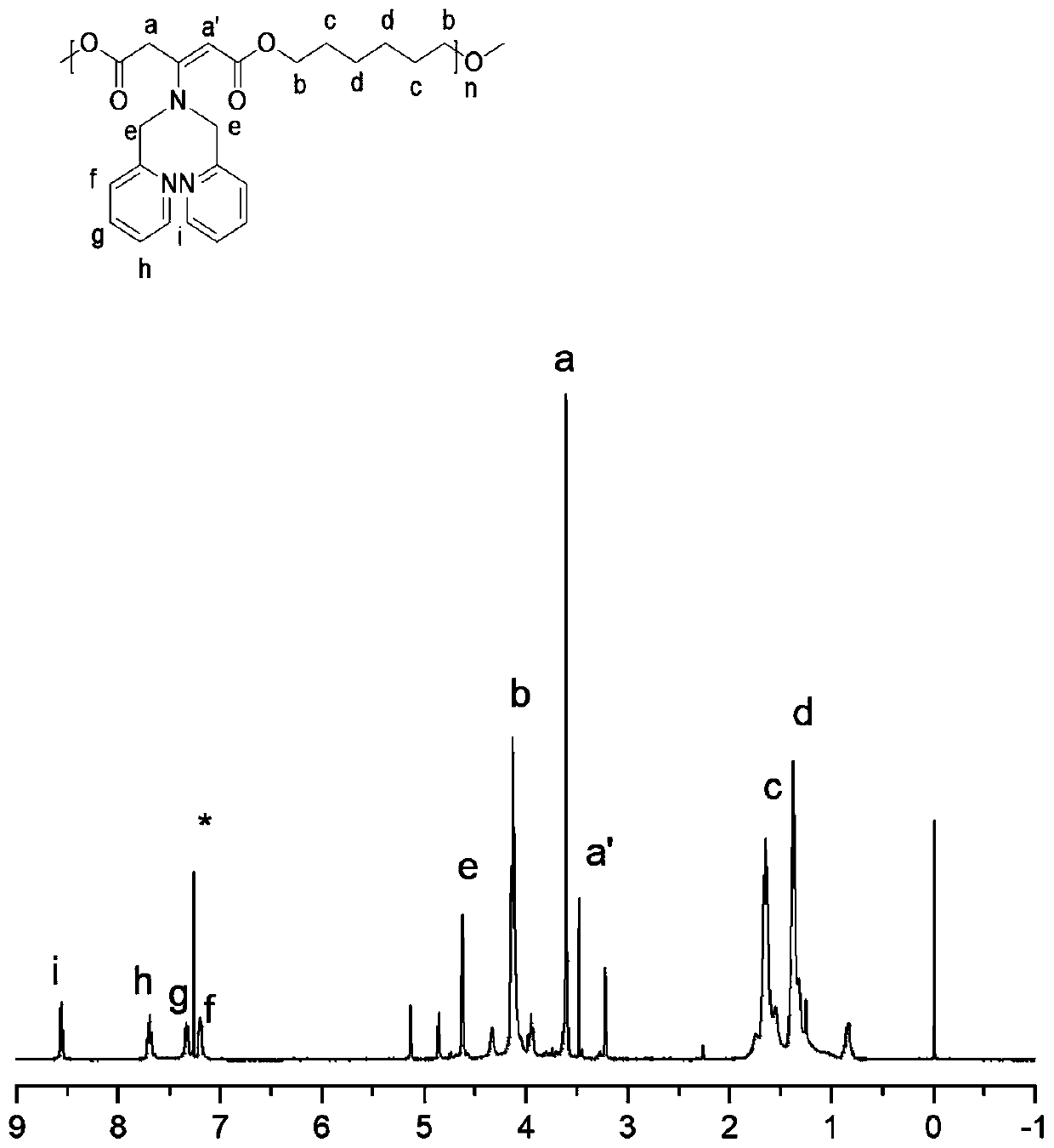
[0028] Taking monomer B as 1,6-hexanediol as an example, calculate: use a precision balance to accurately weigh 54mg of polymer (0.008mmol, carbonyl content is 1eq) in a 20mL reaction bottle, and then accurately weigh 36.6mg (0.184mmol, 1eq ) DPA was added to the reaction bottle, and finally 0.076mg (0.09mmol, 0.05eq) PTSA was weighed, and 2mL DMSO was added as the reaction solvent, and a magnet was added, stirred and heated to 100°C, and reacted for 24h. Stop the reaction, settle in ether for 5 times, remove the reaction raw materials and solvent, and the product obtained by the precipitation is dried in a vacuum oven for 24h, and the nuclear magnetic characterization of the polymer containing DPA in the gained side chain is as follows: figure 2 shown.
Embodiment 3
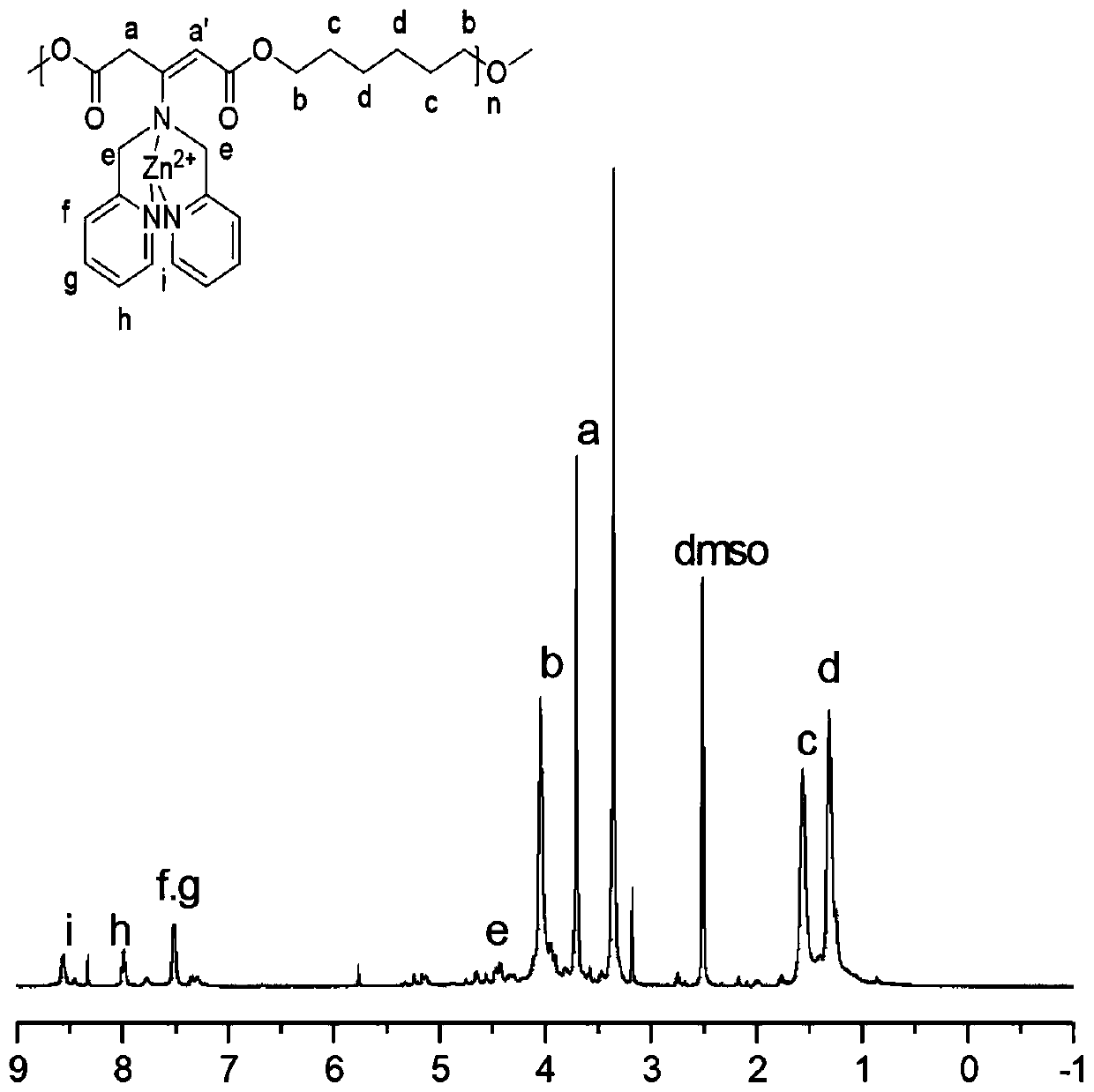
[0030] Taking the monomer B as 1,6 hexanediol as an example to calculate: use a precision balance to accurately weigh 54mg of a polymer containing DPA in the side chain, put it in a 20ml reaction bottle, and then accurately weigh 17mg (0.184mmol) of zinc nitrate, add 1ml of DMSO As the reaction solvent, the reaction was stirred at room temperature for 24h. Stop the reaction, settle in ether for 5 times, remove the reaction raw materials and solvent, and the product obtained by the precipitation is dried in a vacuum oven for 24h, and the nuclear magnetic characterization of the polymer containing DPA-Zn in the gained side chain is as follows: image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com