Preparation method and application of fluorine-containing aromatic ring carboxylic acid tetramer compound
A technology of aromatic ring carboxylic acid and tetramer, which is applied in the application field of anti-neuroblastoma, can solve the problems of rare neuroblastoma, undisclosed inhibition of neuroblastoma, etc., achieve inhibition of cell activity, preparation method Simple, fat-soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
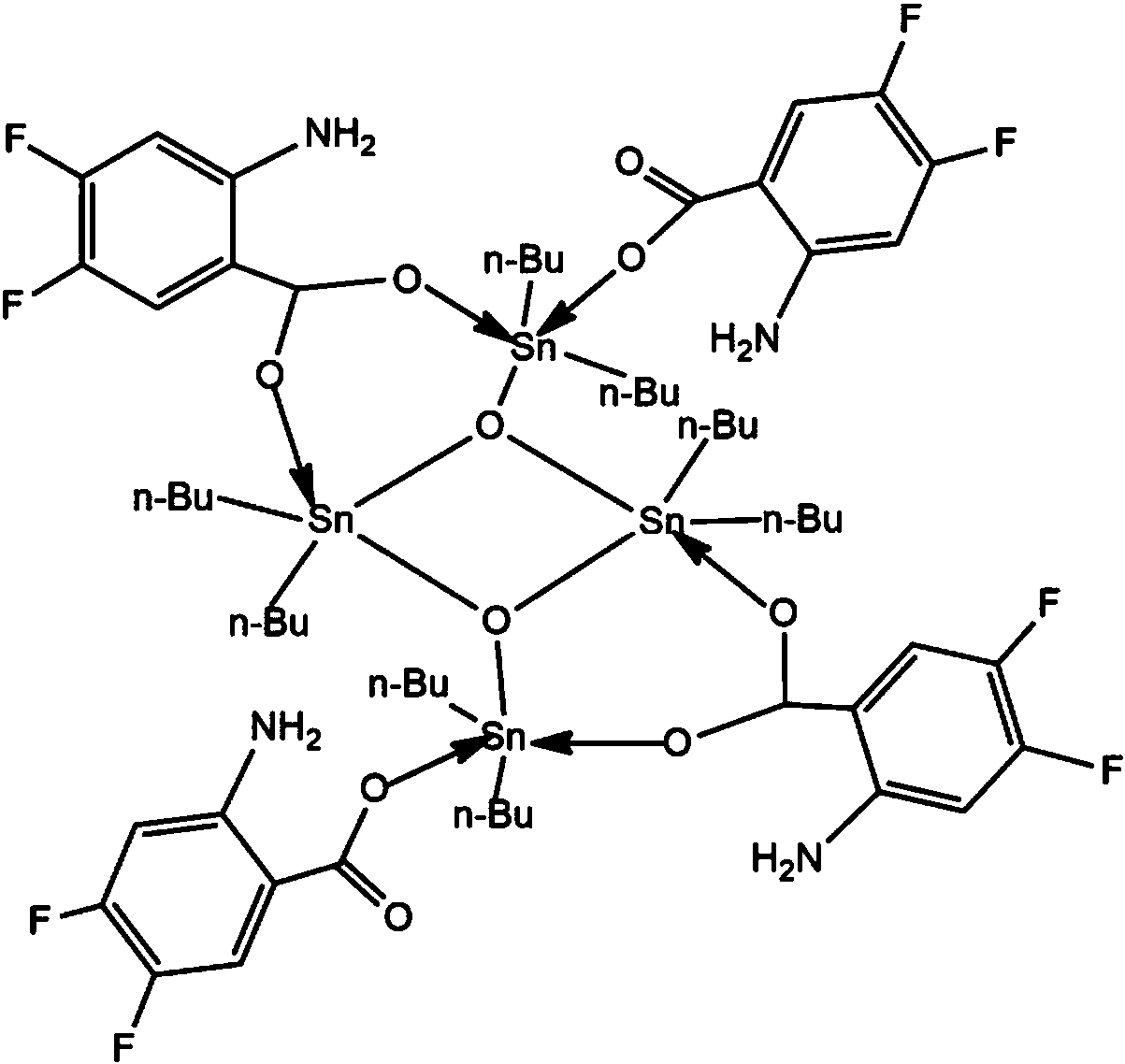
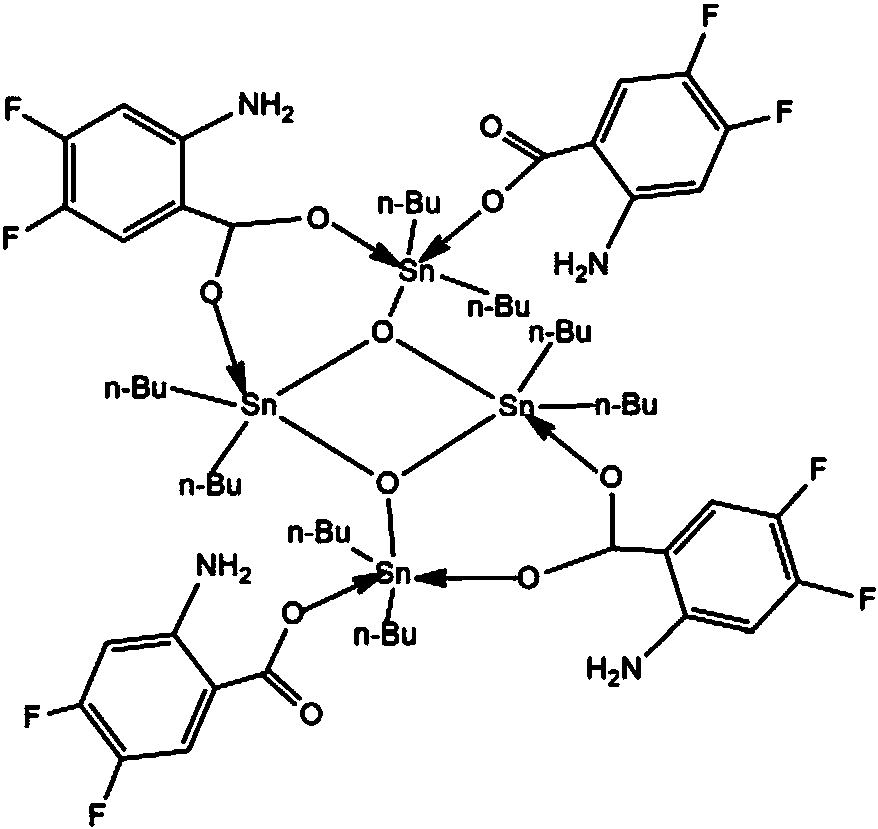
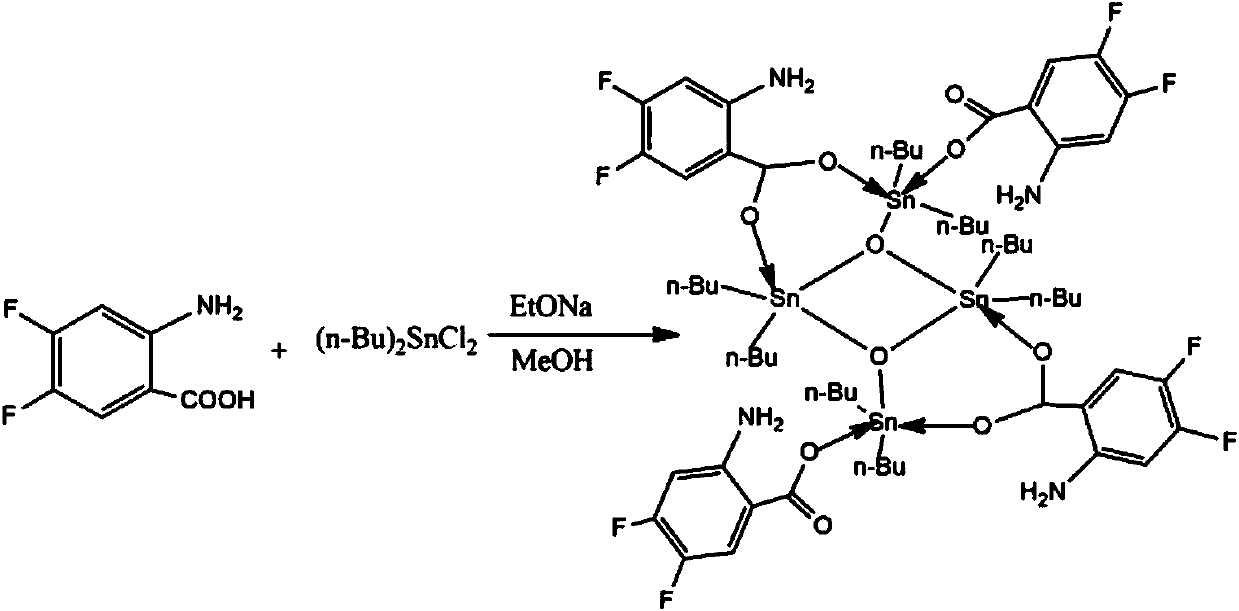
[0021] Preparation of fluorine-containing aromatic ring carboxylic acid tetramer compound: Add 1.0 mmol of 2-amino-4,5-difluoro-benzoic acid, 1.0 mmol of sodium ethoxide, 1.0 mmol of dibutyldichloride to a standard Schlenk tube Chloride tin, 20mL of methanol, stirred at 50°C for 5 hours, and evaporated on a rotary basis to obtain a light yellow powdery solid; recrystallized with dichloromethane-n-hexane to obtain a light yellow transparent crystal, which is the fluorine-containing aromatic ring carboxylic acid tetramer body compound; wherein, the volume ratio of dichloromethane to n-hexane is 6:1. Yield 85%, melting point 112-114°C.
[0022] Through infrared spectrum analysis and elemental analysis and X-ray single crystal diffraction, the results are as follows:
[0023] Infrared spectrum (KBr, cm -1 ): v as (C=O) 1641, 1598; v s (C-O) 1387, 1290; v as (Sn-C)606, v s (Sn-C) 502, v(Sn-O-Sn) 686, 649, v(Sn-O) 479.
[0024] Elemental Analysis: Calculated Value C 60 h 88...
Embodiment 2
[0028] Preparation of fluorine-containing aromatic ring carboxylic acid tetramer compound: add 1.0 mmol of 2-amino-4,5-difluoro-benzoic acid, 1.0 mmol of sodium ethoxide, and 1.0 mmol of dibutyltin dichloride to the flask, 40mL of methanol was stirred at 50°C for 6 hours, and then evaporated to obtain a light yellow solid; recrystallized with dichloromethane-n-hexane to obtain a light yellow transparent crystal, which was a fluorine-containing aromatic ring carboxylic acid tetramer compound; , the volume ratio of dichloromethane to n-hexane is 3:1. Yield 78%, melting point 112-114°C.
Embodiment 3
[0030] Preparation of fluorine-containing aromatic ring carboxylic acid tetramer compound: add 1.0 mmol of 2-amino-4,5-difluoro-benzoic acid, 1.0 mmol of sodium ethoxide, and 1.0 mmol of dibutyltin dichloride to the flask, 60mL of methanol was stirred at 50°C for 5 hours, and then evaporated to obtain a light yellow solid; recrystallized with dichloromethane-n-hexane to obtain a light yellow transparent crystal, which was a fluorine-containing aromatic ring carboxylic acid tetramer compound; , the volume ratio of dichloromethane to n-hexane is 4:1. Yield 82%, melting point 112-114°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com