Preparation method of prothioconazole I-type crystal form
A technology of prothioconazole and crystal form, which is applied in the field of preparation of prothioconazole type I crystal form, can solve the problems of low product yield, poor stability, cumbersome operation, etc., and achieve high purity and crystal form Regular and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of prothioconazole type I crystal form, the specific steps are as follows:
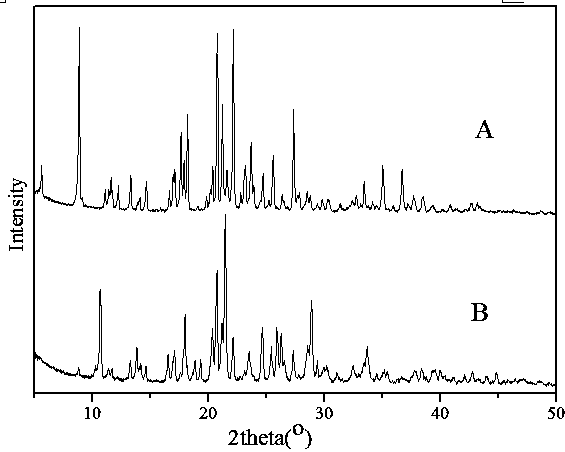
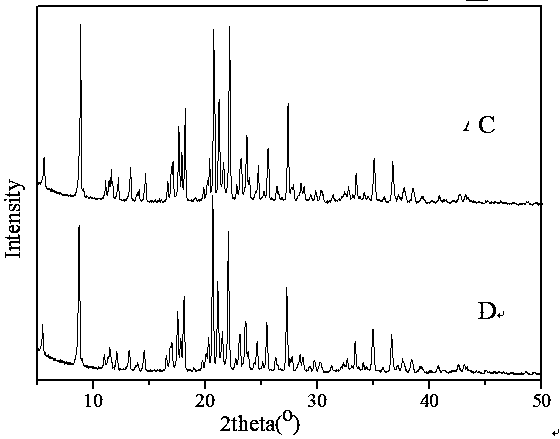
[0028] Put 0.344 g of prothioconazole crude drug in a round bottom flask, add 3ml of toluene into the round bottom flask, heat and stir in a water bath at 50°C for 3 hours, filter while hot, and let the filtrate stand for 3 days after cooling to room temperature to collect the precipitated solid The phase was subjected to negative pressure suction filtration to obtain 0.325 g of type I prothioconazole crystals, the content detected by HPLC analysis: 99.9%, and the melting range: 140.1-140.4°C. The main particle size is 180μm, the bulk density is 0.58g / mL, and the angle of repose is 30°. Use D8 X-ray diffractometer of German BRUKER company to measure, the test conditions are Cu-Kα target tube voltage 40kV, tube current 10mA, scan speed 2° / min, the PXRD diffraction pattern of the product can be found in figure 1 , based on the characteristic reflection, it was confirmed to b...
Embodiment 2
[0030] A preparation method of prothioconazole type I crystal form, the specific steps are as follows:
[0031] Put 0.344 g of a mixture of prothioconazole type I crystals and type II crystals (mass ratio: 2:8) in a round bottom flask, add 3 ml of toluene into the round bottom flask, heat and stir in a water bath at 60°C for 2 hours, and filter while hot , the filtrate was cooled to room temperature and then left to stand for 4 days to collect the precipitated solid phase, and filtered under negative pressure to obtain 0.338 g of type I prothioconazole crystals, the content of which was detected by HPLC analysis: 99.9%, and the melting range: 140.1-140.3°C. The main particle size is 180μm, the bulk density is 0.58g / mL, and the angle of repose is 30°. The spectrum of the product was obtained after PXRD detection, and based on the characteristic reflection, it was confirmed to be the crystal form of prothioconazole I.
Embodiment 3
[0033] A preparation method of prothioconazole type I crystal form, the specific steps are as follows:
[0034]Put 0.344 g of amorphous prothioconazole in a round-bottomed flask, add 3 ml of toluene into the round-bottomed flask, heat and stir in a water bath at 80°C for 2 hours, filter while hot, and let the filtrate stand for 4 days after cooling to room temperature to collect the precipitated The solid phase was filtered under negative pressure to obtain 0.338 g of type I prothioconazole crystals, the content detected by HPLC analysis: 99.9%, and the melting range: 140.0-140.5°C. The main particle size is 180μm, the bulk density is 0.58g / mL, and the angle of repose is 30°. The spectrum of the product was obtained after PXRD detection, and based on the characteristic reflection, it was confirmed to be the crystal form of prothioconazole I.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com