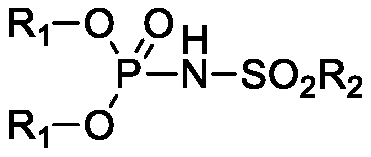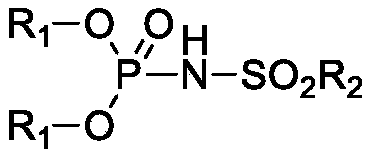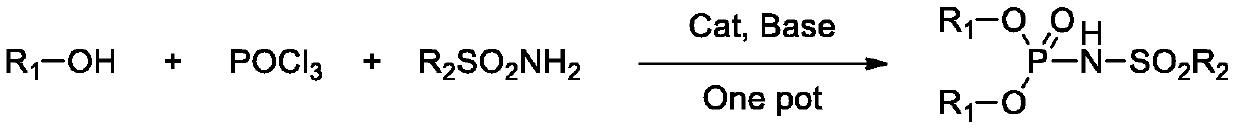Phosphamide derivative as well as preparation method and catalytic application thereof
A technology of phosphoramide and derivatives, which is applied in the catalytic application of phosphoramide derivatives in rearrangement reactions, and in the field of rapid and efficient preparation of phosphoramide derivatives and their one-pot method, which can solve the problems of poor catalyst activity, long reaction time, high Reaction temperature and other issues, to achieve the effect of simplified operation, less dosage, and improved synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of diphenylmethylsulfonylphosphoramidate
[0043] In nitrogen, add 4-dimethylaminopyridine (0.18g, 1.5mmol), dichloroethane (40mL), triethylamine (30.36g, 0.3mol) and phosphorus oxychloride ( 7.67g, 0.05mol), start stirring, put the three-necked bottle into a normal temperature water bath, then slowly add phenol (9.41g, 0.1mol) in dichloroethane (20mL) solution dropwise, after the dropwise addition is complete, continue stirring at room temperature for 2 hours . Finally, a solution of methanesulfonamide (4.76g, 0.05mol) in dichloroethane (20mL) was added dropwise. After the dropwise addition, the water bath was removed, and the three-neck flask was placed in an oil bath, and heated to reflux for 2 hours until the reaction was complete. Post-treatment, the reaction solution was lowered to room temperature, quenched with water, separated phases, and the organic phase was washed 2 to 3 times with 4M hydrochloric acid to remove 4-dimethylaminopyridine and triethy...
Embodiment 2
[0045] Synthesis of diphenyl ethylsulfonylphosphoramidate
[0046] In nitrogen, add 4-pyrrolidinylpyridine (0.37g, 2.5mmol), dichloroethane (40mL), triethylamine (30.36g, 0.3mol) and phosphorus oxychloride ( 7.67g, 0.05mol), start stirring, put the three-neck bottle into a normal temperature water bath, then slowly add phenol (9.65g, 0.1025mol) in dichloroethane (20mL) solution dropwise, after the addition is complete, continue stirring at room temperature for 2 hours . Finally, a solution of ethanesulfonamide (5.46 g, 0.05 mol) in dichloroethane (20 mL) was added dropwise. After the dropwise addition, the water bath was removed, and the three-necked flask was placed in an oil bath, and heated to reflux for 2 hours until the reaction was complete. Post-treatment, the reaction solution was lowered to room temperature, quenched with water, phase separation, the organic phase was washed 2 to 3 times with 4M hydrochloric acid to remove 4-pyrrolidinylpyridine and triethylamine in ...
Embodiment 3
[0048] Synthesis of diphenyl tosylphosphoramidate
[0049] In nitrogen, add 4-dimethylaminopyridine (0.18g, 1.5mmol), dichloroethane (40mL), diisopropylethylamine (45.24g, 0.35mol) and three Phosphorus oxychloride (7.67g, 0.05mol), start stirring, put the three-necked bottle into a normal temperature water bath, then slowly add dropwise a solution of phenol (9.41g, 0.1mol) in dichloroethane (20mL), after the dropwise addition is complete, continue to The reaction was stirred for 2 hours. Finally, a dichloroethane (20 mL) solution of p-toluenesulfonamide (8.56 g, 0.05 mol) was added dropwise. After the dropwise addition, the water bath was removed, and the three-necked flask was placed in an oil bath, and heated to reflux for 2 hours until the reaction was complete. Post-treatment, the reaction solution was lowered to room temperature, quenched with water, separated phases, and the organic phase was washed 2 to 3 times with 4M hydrochloric acid to remove 4-dimethylaminopyridin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com