Preparation method of compound containing C(sp2)-Br bond
A compound, -br technology, applied in the field of organic synthesis, can solve the problems of poor selectivity, using a large amount of oxidants, etc., and achieve the effects of saving production costs, being environmentally friendly, and having high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0076]
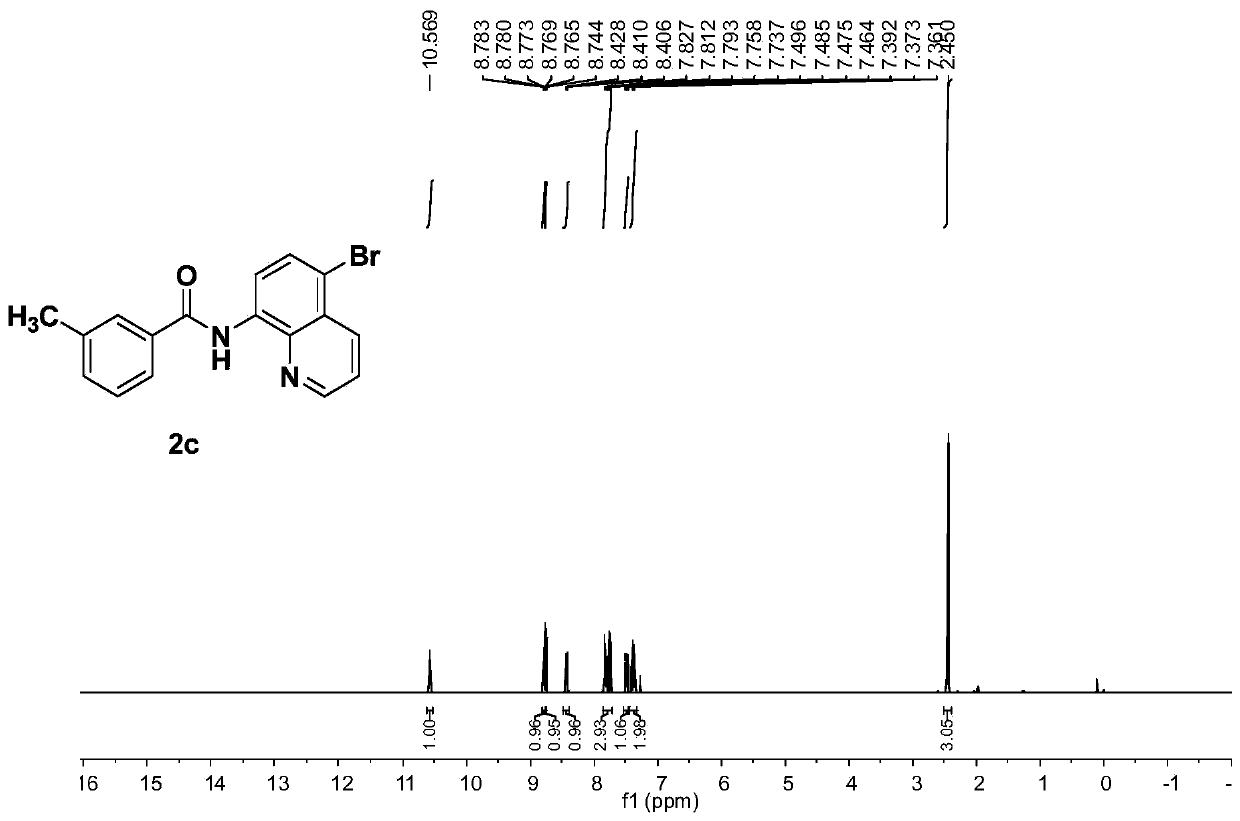
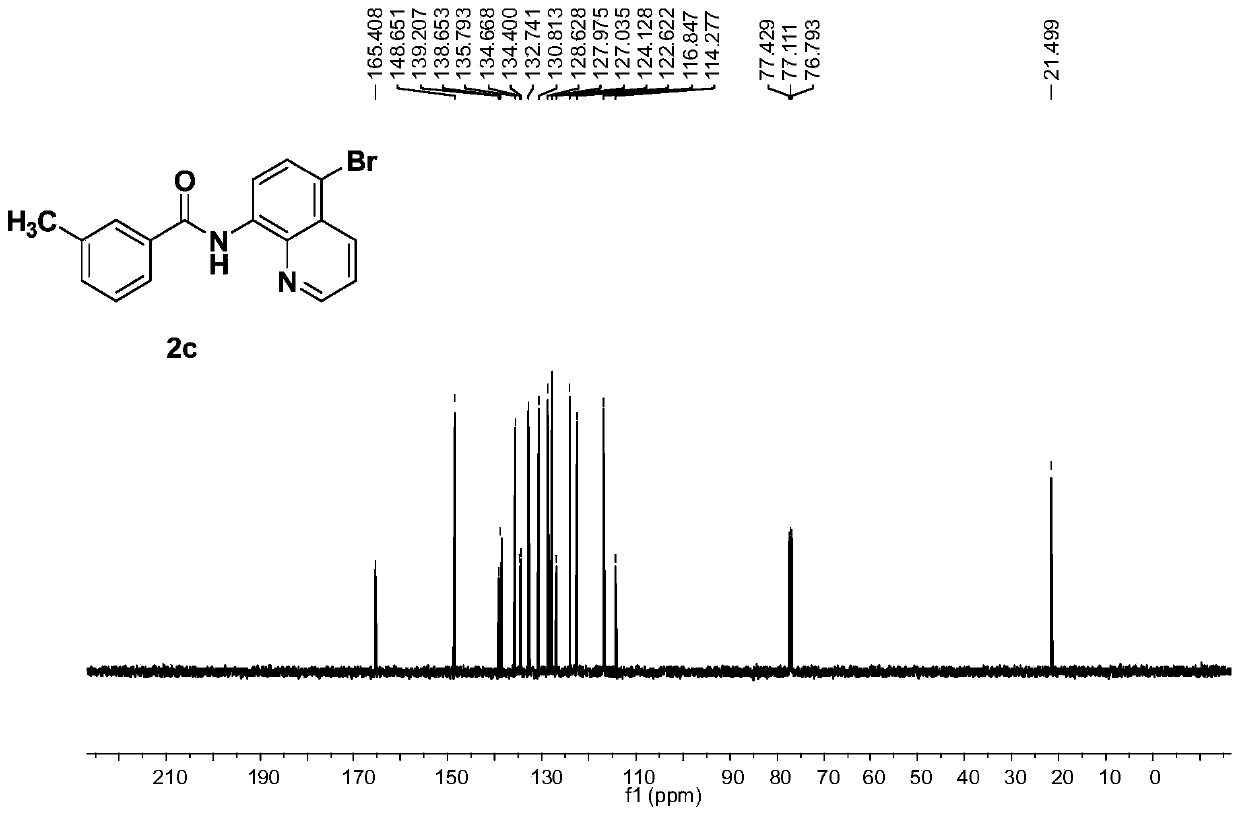
[0077] The electrolysis was carried out in a room equipped with two platinum electrodes (4.0×4.0cm 2 ) in an undivided electrolytic cell. 8-aminoquinoline amide derivative 1c (1.104g, 5.34mmol), NH 4 Br(6.23g, 64.08mmol), Cu(OAc) 2 (96.73 mg, 0.534 mmol), DMF (80 mL) was added to the electrolytic cell. Electrolysis was carried out at 60 °C with a constant current of 20 mA for 80 h. After the reaction was complete, it was quenched with ice-cold water and extracted with EtOAc. The combined organic extracts were dried over anhydrous sodium sulfate and filtered, and the solvent was evaporated under reduced pressure to obtain a crude product, which was separated and purified by silica gel column chromatography to obtain a white solid 3c (1.704 g, 93%, purity greater than 95%). 1 H NMR (400MHz, CDCl 3 )δ10.57(s,1H),8.78(dd,J=4.2,1.2Hz,1H),8.75(d,J=8.4Hz,1H),8.42(dd,J=8.6,1.2Hz,1H), 7.84-7.73(m,3H),7.48(dd,J=8.2,4.4Hz,1H),7.42-7.32(m,2H),2.45(s,3H). 13 C NMR (100MH...
Embodiment 1-2
[0079]
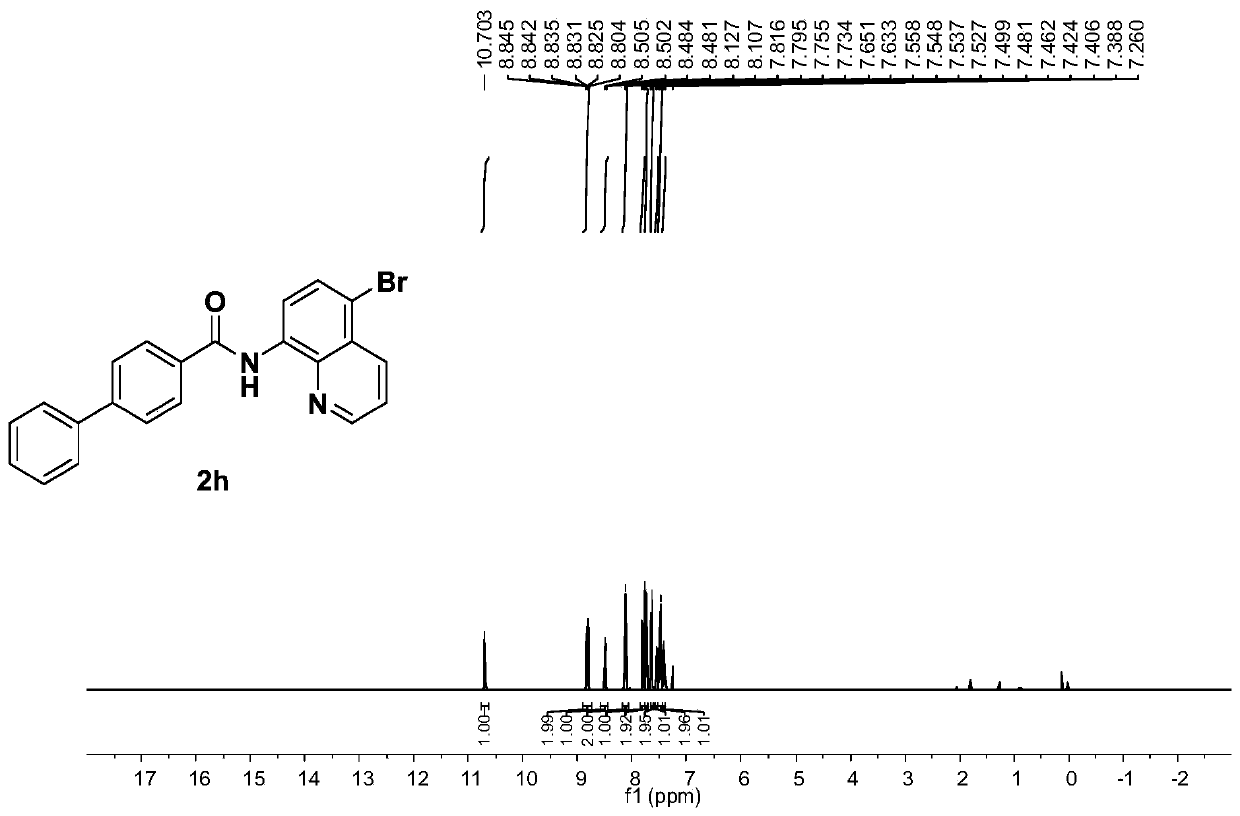
[0080] The electrolysis was carried out in a cell equipped with two platinum electrodes (1.0×1.0cm 2 ) in an undivided electrolytic cell. 8-aminoquinoline amide derivative 1h (64.8mg, 0.2mmol), NH 4 Br(232.8mg, 2.4mmol), Cu(OAc) 2 (3.62mg, 0.02mmol), DMF (3mL) was added to the electrolytic cell. The electrolysis was performed at 60 °C for 34 h at a constant current of 3 mA. The progress of the reaction was monitored by TLC. After the reaction was complete, it was quenched with ice-cold water and extracted with EtOAc. The combined organic extracts were dried over anhydrous sodium sulfate and filtered, and the solvent was evaporated under reduced pressure to obtain a crude product, which was separated and purified by silica gel column chromatography to obtain a white solid 2h (79.3 mg, 98%, purity greater than 95%). 1 H NMR (400MHz, CDCl 3 ):δ10.70(s,1H),8.84(dd,J=4.0,1.2Hz,1H),8.81(d,J=8.4Hz,1H),8.49(dd,J=8.4,1.2Hz,1H) ,8.12(d,J=8.0Hz,2H),7.81(d,J=8.0Hz,1H),7...
Embodiment 1-3
[0082]
[0083] Using the same reaction conditions as above, starting from 1i (59.6 mg, 0.25 mmol), electrolysis for 33 h gave 2i (71.0 mg, 94%, purity greater than 95%) as a white solid. 1 H NMR (400MHz, CDCl 3 ):δ10.40(s,1H),8.94(d,J=8.4Hz,1H), 8.75(dd,J=4.4,1.6Hz,1H),8.56-8.50(m,2H),8.01(d, J=8.4Hz,1H),7.95-7.86(m,3H), 7.62-7.51(m,4H); 13 C NMR (101MHz, CDCl 3 ): δ167.68, 148.78, 139.26, 135.97, 134.69, 134.29, 133.86, 131.34, 130.93, 130.27, 128.44, 127.41, 127.25, 126.58, 125.56, 125.45, 112.74.16, 9.12 1 H NMR spectrum as Figure 5 shown; 13 C NMR spectrum as Figure 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com