Folic acid modified ABT-737 loaded mesoporous silica nanoparticles, and preparation method thereof
A technology of ABT-737 and mesoporous silica, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve low bioavailability, poor solubility of ABT-737, etc. problem, to achieve good killing effect, improve bioavailability, and obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of carrying ABT-737 mesoporous silica nanoparticles, comprising:
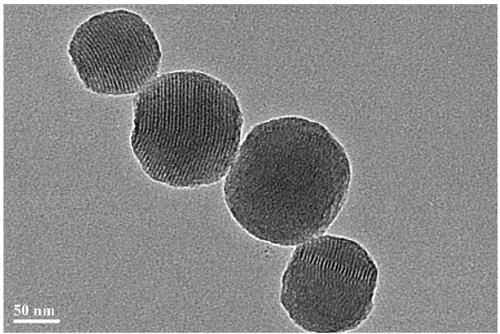
[0029] (1) Preparation of FA-MSN: 200 mg of folic acid was dissolved in 12 mL of dimethyl sulfoxide, reacted with 180 mg of NHS (N-hydroxysuccinimide) and 100 mg of DCC (dicyclohexylcarbodiimide) at room temperature for 10 h , centrifuged to collect the precipitate, washed and freeze-dried. The activated folic acid was dissolved in 5 mL of dimethyl sulfoxide, and 350 mg of NH 2 -PEG-NH 2 , stirred at room temperature, reacted for 22 hours, centrifuged, and freeze-dried to obtain functionalized folic acid NH 2 -PEG-FA.
[0030] Another 50mg of carboxyl-modified silica mesoporous nanoparticles (MSN / COOH) with a particle size of about 180nm was dispersed in 2-(N-morpholine)ethanesulfonic acid buffer (0.1M, pH=5), and 70mg of EDAC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride) reacted with 35mg of NHS for 1.5h, centrifuged, and added 45mg of NHS 2 -PEG-FA, stirred ...
Embodiment 2
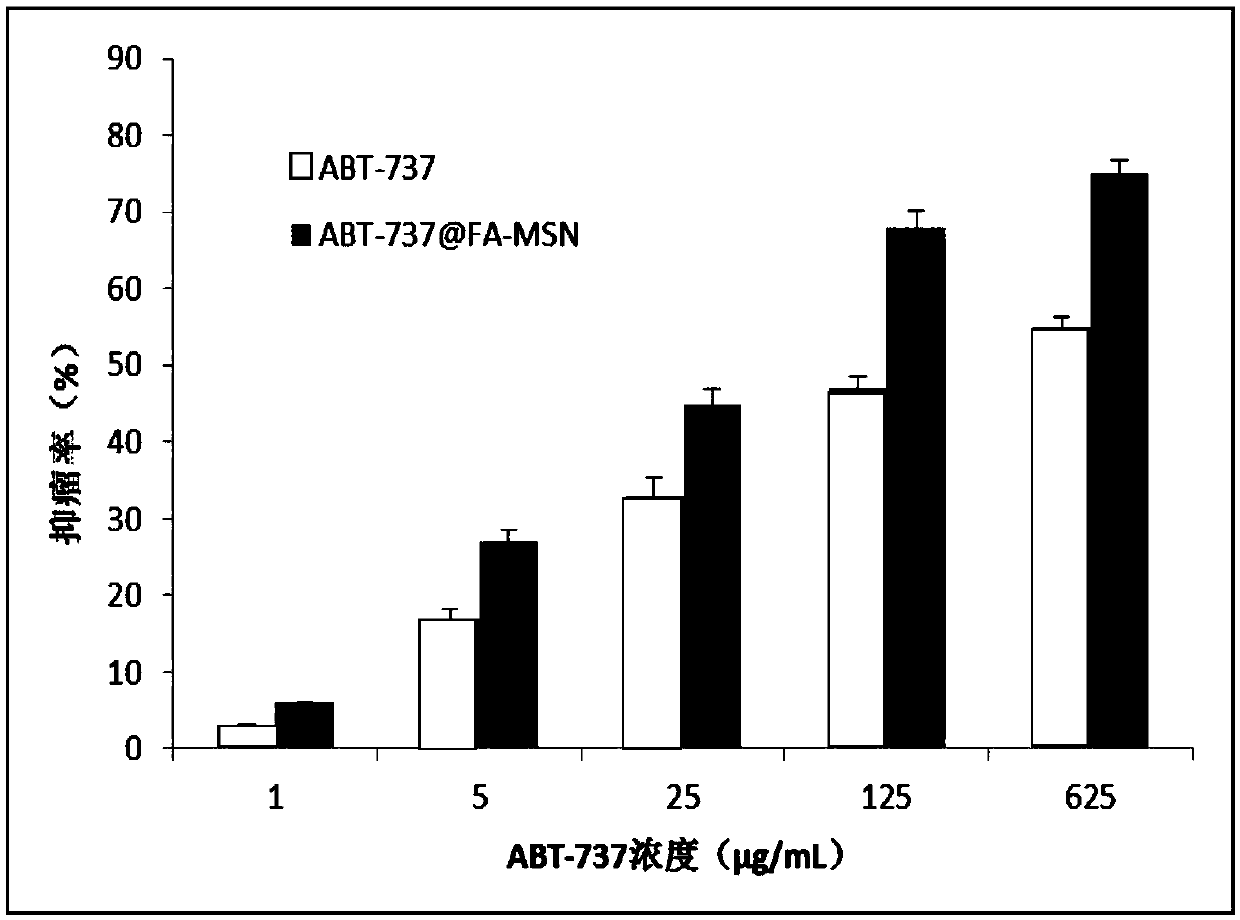
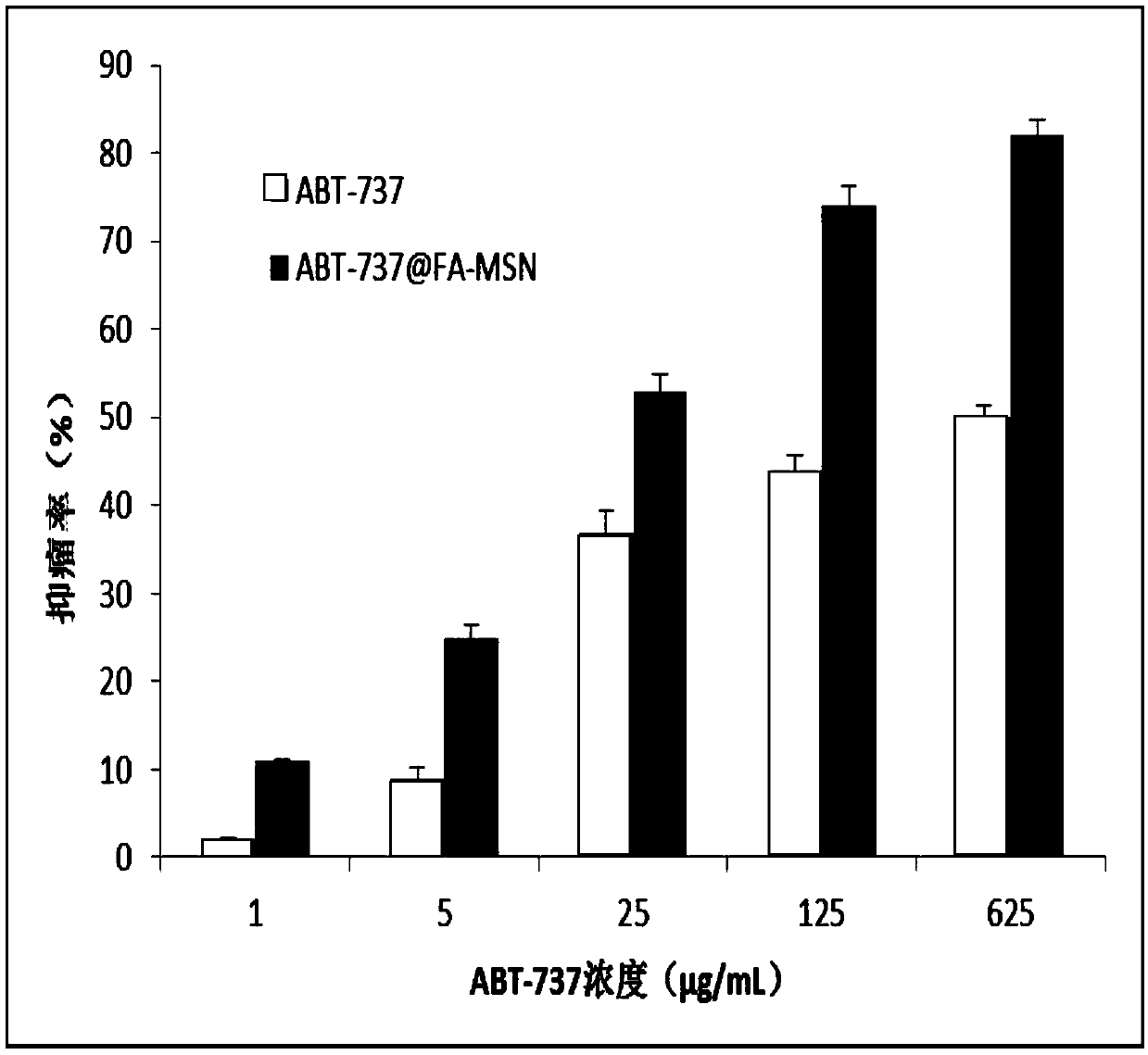
[0046] H22 in vitro cytotoxicity test
[0047] Take the H22 in the logarithmic growth phase, collect the suspended cell liquid, adjust the cell concentration, and inoculate in a 96-well plate at a seeding density of 1×10 4 / well; after the 96-well plate was incubated in a constant temperature incubator for 24 hours, the original drug (ABT-737) and the ABT-737@FA-MSN group were treated with different concentrations (1, 5, 25, 125, 625 μg / mL, (based on the weight of ABT) was added, and three replicate wells were set up for each concentration to calculate the average value; 0.2 mL of sterilized PBS buffer solution was added to the outermost well of the 96-well plate to resist the edge effect; the 96-well plate was respectively Continue to incubate for 24 and 48 hours, take a 96-well plate according to the time point, add 20 μL / well of 5 mg / ml MTT solution, and continue to incubate at 37°C for 4 hours; carefully suck out the liquid in the well, then add DMSO, 100 μL / well; The ins...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com