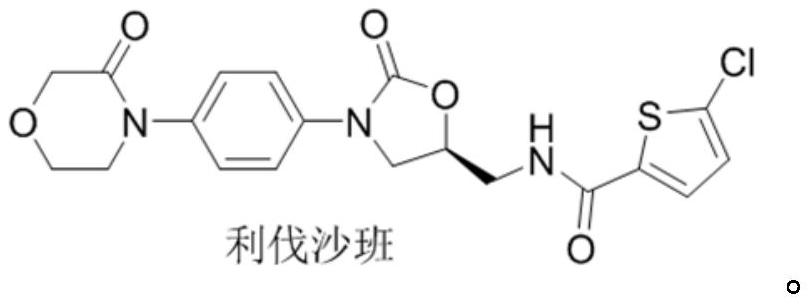
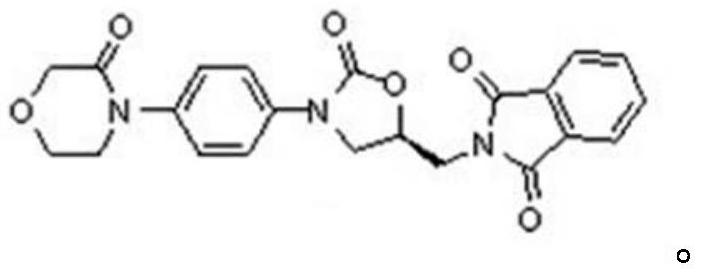
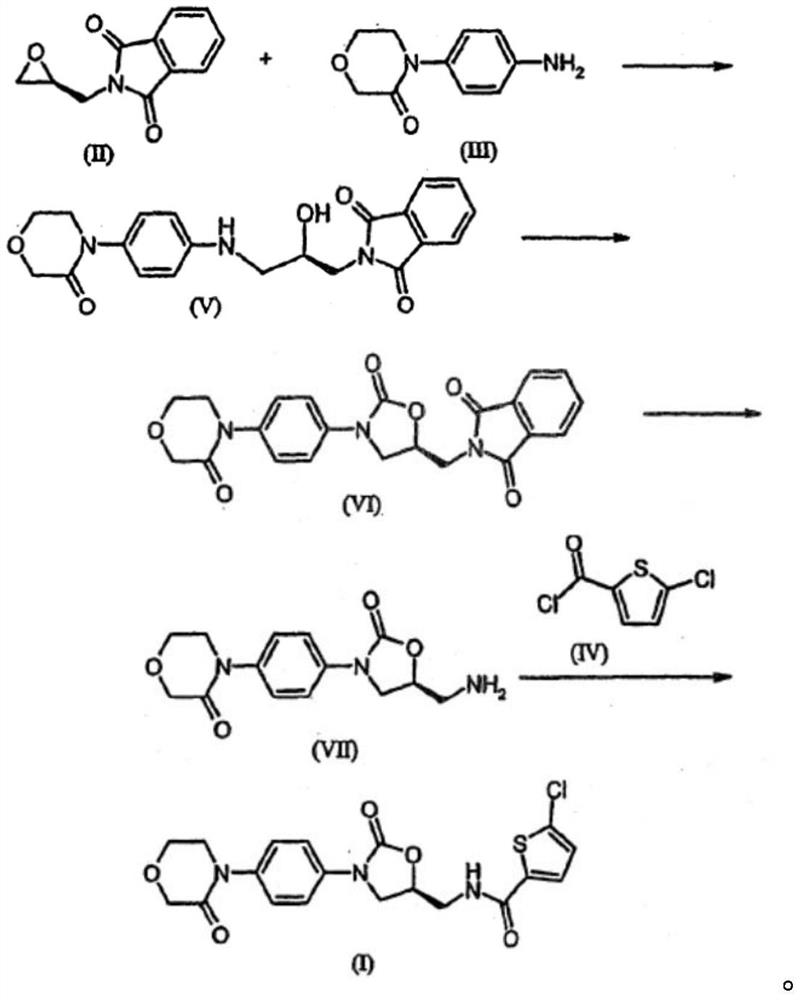
A kind of preparation method of rivaroxaban intermediate
A technology for rivaroxaban and intermediates, applied in the field of preparation of rivaroxaban intermediates, can solve the problem that the purity and impurity level cannot meet the quality requirements of raw material drug intermediates, affect the quality of rivaroxaban, and the compound is not easy to obtain and other problems, to achieve the effect of strong impurity removal ability, good process tolerance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of compound B
[0058] Add 92.6g of phthalimide potassium salt into a 1000mL reaction flask, add 400mL of (S)-epichlorohydrin, raise the temperature to 70°C, react for 1 hour, concentrate and recover the solvent, and wash the residue with 500mL of absolute ethanol Recrystallized to obtain 84g of compound B, HPLC purity: 95.1%, molar yield: 83%, ee value: 96.0%.
[0059] NMR data: 1 H-NMR (DMSO-d6) δ (ppm): 2.59 (1H, m), 2.76 (1H, m), 3.22 (1H, m), 3.78 (2H, d, J = 4.56Hz), 7.86 (2H, m),7.89(2H,m)
Embodiment 2
[0060] Embodiment 2: the preparation of compound B
[0061] Add 92.6g of phthalimide potassium salt into a 1000mL reaction flask, add 600mL of (S)-epichlorohydrin, raise the temperature to 40°C, react for 3 hours, concentrate and recover the solvent, and wash the residue with 500mL of absolute ethanol Recrystallization, recrystallization twice (500mL×2), obtained 65g of compound B, HPLC purity: 98.2%, molar yield: 64%, ee value: 97.5%.
[0062] The NMR data of Compound B prepared in Example 2 is consistent with the NMR data of Example 1.
Embodiment 3
[0063] Embodiment 3: the preparation of compound B
[0064] Add 92.6g of phthalimide potassium salt into a 1000mL reaction flask, add 600mL of (S)-epichlorohydrin, raise the temperature to 40°C, react for 3 hours, concentrate and recover the solvent, and wash the residue with 500mL of absolute ethanol Recrystallized once, and then recrystallized once with 200 mL of ethyl acetate to obtain 53 g of compound B, HPLC purity: 99.8%, molar yield: 52%, ee value: 98.5%.
[0065] The NMR data of Compound B prepared in Example 3 is consistent with the NMR data of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com