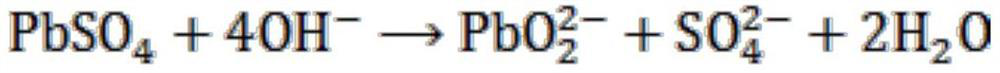A kind of recovery method of waste lead-acid battery
A recycling method, waste lead-acid technology, applied in the direction of battery recycling, lead-acid storage battery, recycling of waste collectors, etc., can solve the problems of waste lead-acid battery recycling method is not clean enough, to achieve more atomic economy, broad market prospects, The effect of simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
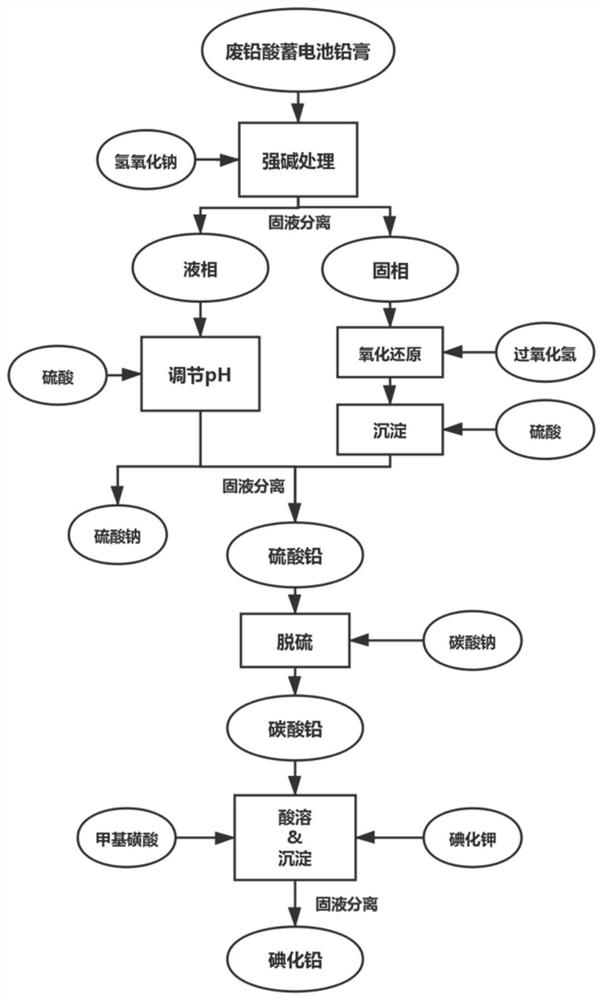
[0097] Take 100g of the lead paste obtained from the spent lead-acid battery, use 1L of NaOH solution with a concentration of 2mol / L to leach it, stir it thoroughly for 1h, and then filter it. The filter residue that obtains in the above-mentioned filtration process first takes the H that pH is 2 0.5mol / L 2 o 2 Solution 500mL is reacted (using methanesulfonic acid to adjust the pH value of the solution), then to the filtrate obtained by filtering after the reaction, add 150mL of sulfuric acid aqueous solution with a concentration of 2mol / L and stir, filter, wash and dry again to obtain high-purity lead sulfate . And the filtrate obtained after the above-mentioned NaOH solution leaching lead plaster and solid-liquid separation is added dropwise concentrated sulfuric acid and fully stirred to adjust the pH value of the solution to about 3, and then filtered, washed and dried to obtain high-purity sulfuric acid. lead. After the lead sulfate obtained in the above two different ...
Embodiment 2
[0100] Take 250g of the lead paste obtained from the spent lead-acid battery, use 3L of NaOH solution with a concentration of 2mol / L to leach it, stir it thoroughly for 1h and 20min, and then filter it. The filter residue that obtains in the above-mentioned filtering process first takes the H of 0.5mol / L that pH is 1.5 2 o 2 Solution 1.5L is reacted (using methanesulfonic acid to adjust the pH value of the solution), and then to the filtrate obtained by filtering after the reaction, add 500mL of sulfuric acid aqueous solution with a concentration of 2mol / L and stir, and then filter, wash and dry to obtain high-purity sulfuric acid lead. And the filtrate obtained after the above-mentioned NaOH solution leaching lead plaster and solid-liquid separation is added dropwise concentrated sulfuric acid and fully stirred to adjust the pH value of the solution to about 3, and then filtered, washed and dried to obtain high-purity sulfuric acid. lead. After the lead sulfate obtained in...
Embodiment 3
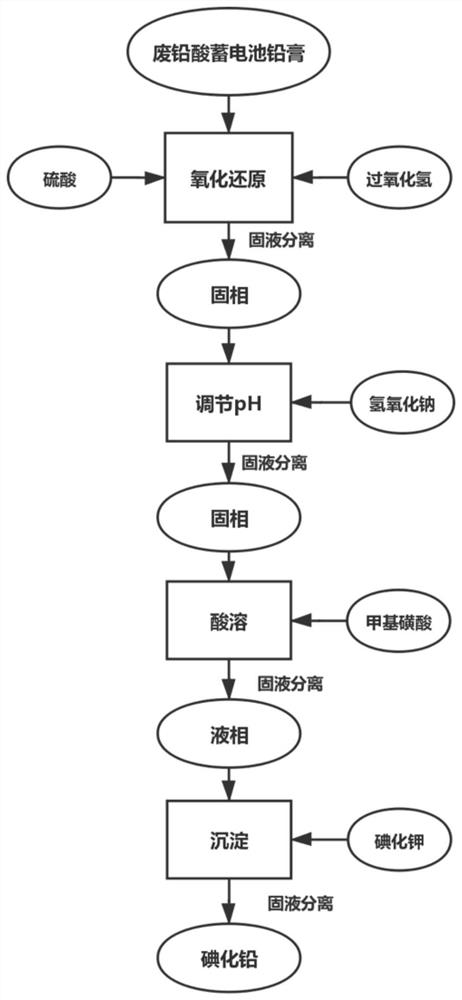
[0103]Take 100g of lead paste obtained from spent lead-acid batteries, add 250mL of sulfuric acid with a concentration of 2mol / L and 100mL of aqueous hydrogen peroxide solution with a concentration of 1mol / L to it, and dilute it to 1000ml of aqueous solution, and leaching it under stirring conditions. 1h. After solid-liquid separation, add 2L sodium hydroxide solution with a concentration of 0.5mol / L to the filter residue, adjust the pH to about 8 with sulfuric acid, leaching for 1 hour under stirring conditions, filter and separate, and add 400mL of sodium hydroxide solution with a concentration of 0.5mol / L to the white precipitate. 2.1mol of methanesulfonic acid was stirred continuously under the condition of operating temperature of 60°C. After the white precipitate was completely dissolved, the concentration was adjusted to obtain 1mol / L lead methanesulfonate, and then 400mL of lead methanesulfonate with a concentration of 2.2mol / L was added. Potassium iodide solution was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com