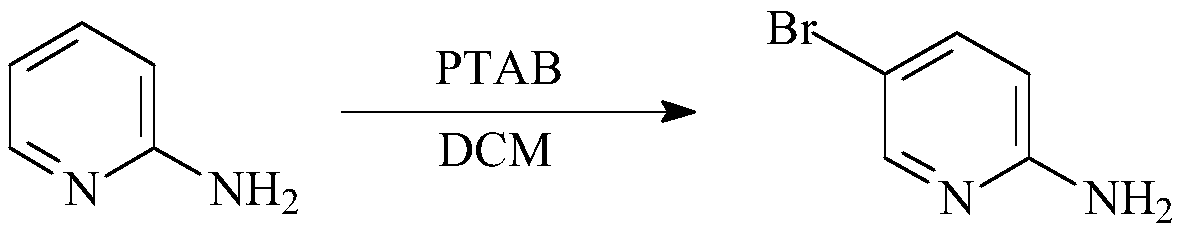
Preparation method of 2-amino-5-bromopyridine
A technology of aminopyridine and bromopyridine, which is applied in the field of preparation of 2-amino-5-pyridine, can solve the problems of many 3-position by-products, expensive brominating agent, corrosiveness, etc., and achieve low cost and mild reaction conditions , the effect of reducing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 9.4g of 2-aminopyridine (0.1mol), 37.6g of phenyltrimethylammonium tribromide (0.1mol) and 300ml of chloroform into a 1L three-necked flask, insert a mechanical stirrer, a thermometer and a condensing reflux tube into the three-necked flask , start stirring to make it evenly mixed, stir at 30°C for 2 hours, wash with 40ml of saturated sodium chloride solution prepared in advance, the water phase is in the upper layer, and the organic phase is in the lower layer. Wash with water 2-3 times, separate the layers, dry with anhydrous sodium sulfate, filter, and remove the solvent chloroform by rotary evaporation of the organic phase to obtain an oil, cool with ice water, add water to precipitate a solid, and obtain a crude product, recrystallize with benzene, filter, After drying, 10 g of yellow solid was obtained with a yield of 81%.
Embodiment 2
[0020] Add 9.4g of 2-aminopyridine (0.1mol), 37.6g of phenyltrimethylammonium tribromide (0.1mol) and 300ml of chloroform into a 1L three-necked flask, insert a mechanical stirrer, a thermometer and a condensing reflux tube into the three-necked flask , start stirring to make it evenly mixed, stir at 25°C for 2 hours, wash with 40ml of saturated sodium chloride solution prepared in advance, the water phase is in the upper layer, and the organic phase is in the lower layer. Wash with water 2-3 times, separate the layers, dry with anhydrous sodium sulfate, filter, and remove the solvent chloroform by rotary evaporation of the organic phase to obtain an oil, cool with ice water, add water to precipitate a solid, and obtain a crude product, recrystallize with benzene, filter, After drying, 8.43 g of yellow solid was obtained, with a yield of 78%.
[0021] This embodiment is the best implementation mode.
Embodiment 3
[0023] Add 9.4g of 2-aminopyridine (0.1mol), 37.6g (0.1mol of phenyltrimethylammonium tribromide) and 300ml of dichloromethane into a 1L three-necked flask, insert a mechanical stirrer, a thermometer and a condenser in the three-necked flask Return the tube, start stirring to make it evenly mixed, stir at 30°C for 2 hours, wash with 40ml of saturated sodium chloride solution prepared in advance, the water phase is in the upper layer, and the organic phase is in the lower layer. Wash with 20ml of water for 2-3 times, separate layers, dry with anhydrous sodium sulfate, filter, and remove the solvent chloroform by rotary evaporation of the organic phase to obtain an oil, cool with ice water, add water to precipitate a solid, obtain a crude product, recrystallize with benzene, After filtering and drying, 8.1 g of yellow solid was obtained, with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com