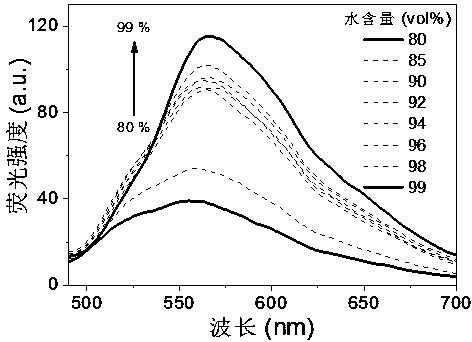
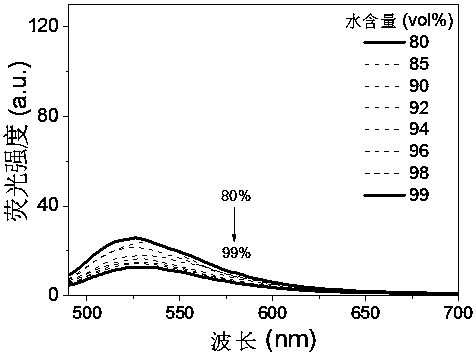
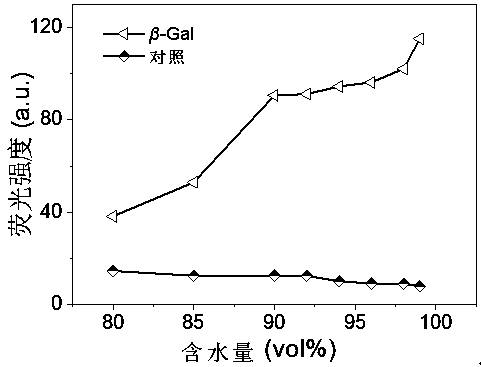
Cyano vinylene derivative fluorescent dye as well as preparation method and application thereof
A compound, triethylamine technology, used in the field of chemical sensors, can solve the problems of photophysical properties restricting development and application, and achieve the effect of significant effect and high sensitivity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] (1) Weigh 2-benzimidazole acetonitrile (266.0 mg, 1.70 mmol) and 4-(N,N-diethyl)aminobenzaldehyde to dissolve in ethanol (20 mL), then add piperidine (1.0 mL, 17.0 mmol), stirred at room temperature for 4 h under the protection of inert gas. After the reaction, a large amount of homogeneous orange-yellow fine sand-like precipitates were formed in the solution. The reaction solution was filtered, washed with ice ethanol, and the filter residue was dried to obtain an orange-yellow powder solid, which was the relatively pure product 3 (396.0 mg, 72%); 1 H NMR (400 MHz, DMSO-d 6 ): δ = 12.79 (s, 1H), 8.11 (s, 1H), 7.90 (d, 2H, J = 8.4 Hz),7.63 (d, 1H, J = 7.2 Hz), 7.50 (d, 1H, J = 7.2 Hz), 7.20 (m, 2H, J = 7.2 Hz),6.84 (d, 2H, J = 8.4 Hz), 3.46 (q, 4H, J = 7.2 Hz), 1.14 (t, 6H, J = 6.4 Hz); 13 C NMR (100 MHz, DMSO-d 6 ): δ = 150.6, 149.5, 145.8, 144.0, 135.3, 132.8, 123.2, 122.3, 119.6, 119.0, 118.4, 111.8, 111.6, 93.4, 44.4, 12.9; HRMS (E...
Embodiment 2
[0038] Example 2 Preparation of Compound 12
[0039] The preparation method of compound 3 is the same as that in Example 1.
[0040]
[0041] (1) Weigh p-Hydroxybenzaldehyde 6 (257.0 mg, 2.10 mmol) and dissolve it in 1 mol / L sodium hydroxide solution, add 2, 3, 4, 6-tetraacetyl in acetone dropwise - β -D-Glucopyranose 7 (867.0 mg, 2.10 mmol), the reaction was stirred at reflux (60 ± 3 °C) for 4 h. When the solution turned yellow completely, the solvent was removed by distillation under reduced pressure after the reaction. The obtained solid was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether = 1 / 3) to obtain compound 8 (598.0 mg, 63%); 1 H NMR (400 MHz, CDCl 3 ) : δ = 9.92 (s, 1H), 7.86 (d, 2H, J = 8.8 Hz),7.12 (d, 2H, J = 8.0 Hz), 5.51 (d, 2H, J = 17.6 Hz), 5.20 (d, 1H, J = 8.0Hz), 5.15 (d, 1H, J = 9.6 Hz), 4.16 (m, 3H), 2.195 (s, 3H), 2.07 (s, 6H),2.026 (s, 3H). HRMS (ESI): calcd for C 21 h 25 o 11 [M+H] + m / z 4...
Embodiment 3
[0051] Using a method similar to Example 2, those skilled in the art can easily prepare R 1 independently selected from NH 2 , OH, CN, CH 3 , COOH, SO 3 H, F, Cl, Br or NO 2 , R 2 for compound of.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com