Mn<4+>-doped alkali metal fluoro-phosphate red luminescent material and preparation method thereof
A technology of fluorophosphate and red luminescence, which is applied in the direction of luminescent materials, chemical instruments and methods, and can solve the problems of limited application prospects, expensive production raw materials, and harsh synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 11.14 g of cesium fluoride and add to 10 ml (40 %) hydrofluoric acid (HF) and 2.2 ml of hexafluorophosphoric acid to react for 30 minutes. Then, 0.15 g of potassium hexafluoromanganate was added to the solution to continue the reaction for 60 minutes. The obtained solid precipitate was washed 5 times with acetic acid, and then the solid precipitate was dried in a vacuum oven for 24 hours, and finally the obtained pink powder was the final product CsPF 6 :Mn 4+ .
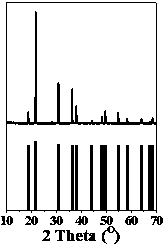
[0022] attached figure 1 Shown is the XRD diffraction pattern of this luminescent material, which is consistent with the standard card JCPDS 34-0506 (CsPF 6 ) are basically consistent, which indicates that the structure of our synthesized sample is similar to that of CsPF 6 unanimous.
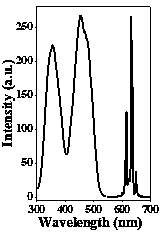
[0023] attached figure 2 Shown are the room temperature excitation spectrum (monitored at 633 nm) and emission spectrum (excited at 455 nm) of the sample. The sample has strong broadband excitation in the blue lig...
Embodiment 2
[0027]Weigh 7.82 g of rubidium fluoride and add to 10 ml (40%) hydrofluoric acid (HF) and 2.2 ml hexafluorophosphoric acid to react for 60 minutes. Then, 0.15 g of potassium hexafluoromanganate was added to the solution to continue the reaction for 60 minutes. The obtained solid precipitate was washed 4 times with acetic acid, and then the solid precipitate was dried in a vacuum oven for 24 hours, and finally the obtained pink powder was the final product RbPF 6 :Mn 4+ .
[0028] attached Figure 5 Shown is the XRD diffraction pattern of this luminescent material, which is consistent with the standard card JCPDS 32-0936 (RbPF 6 ) are basically consistent, which shows that the structure of our synthesized sample is consistent with that of RbPF 6 unanimous.
[0029] attached Figure 6 Shown is sample RbPF 6 :Mn 4+ The room temperature excitation spectrum (monitoring wavelength is 632 nm) and emission spectrum (excitation wavelength is 452 nm). The sample has strong broa...
Embodiment 3
[0032] Weigh 7.82 g of potassium fluoride and add to 10 ml (40%) hydrofluoric acid (HF) and 2.2 ml of hexafluorophosphoric acid to react for 60 minutes. Then, 0.15 g of potassium hexafluoromanganate was added to the solution to continue the reaction for 120 minutes. The obtained solid precipitate was washed 5 times with acetic acid, and then the solid precipitate was dried in a vacuum oven for 24 hours, and finally the obtained pink powder was the final product KPF 6 :Mn 4+ .
[0033] attached Figure 8 Shown is the XRD diffraction pattern of this luminescent material, which is consistent with the standard card JCPDS 52-1826 (KPF 6 ) are basically consistent, which shows that the structure of our synthesized sample is consistent with that of KPF 6 unanimous.
[0034] attached Figure 9 Shown are the room temperature excitation spectrum (monitored at 630 nm) and emission spectrum (excited at 464 nm) of the sample. The sample has strong broadband excitation in the blue li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com