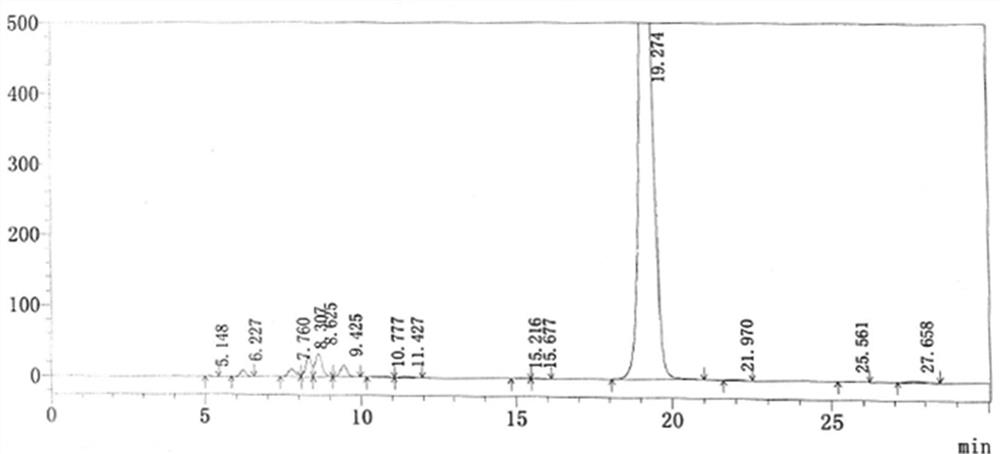
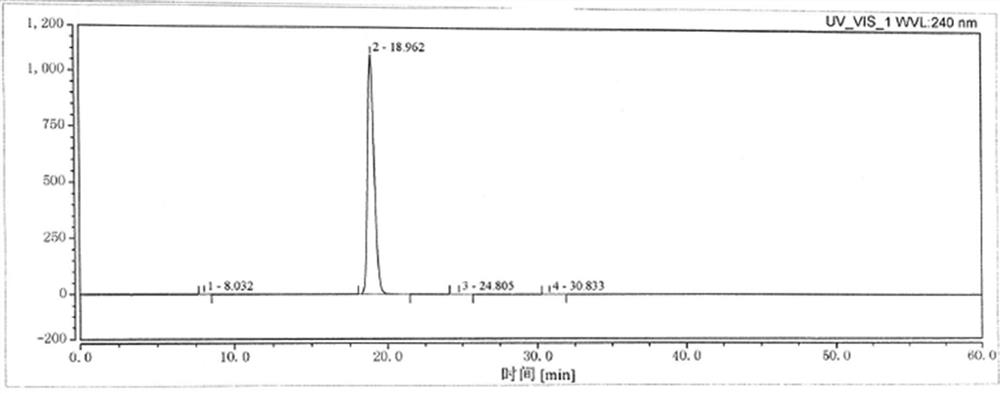
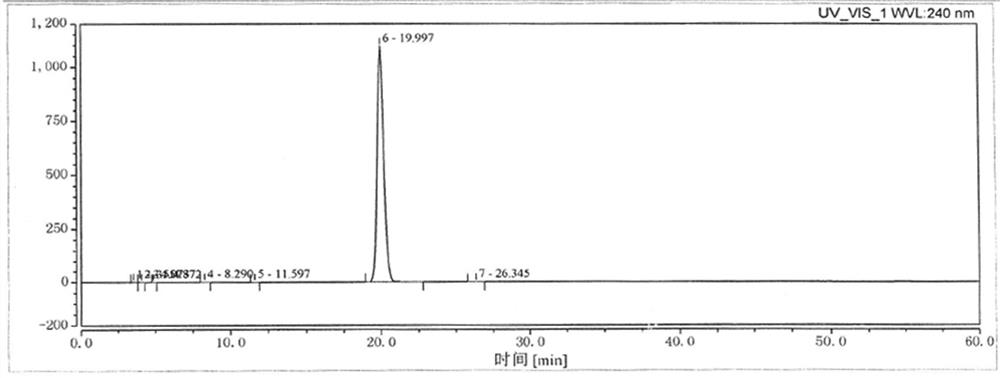
The preparation method of cilnidipine
A technology of cilnidipine and acetyl, which is applied in the field of high-purity cilnidipine compound preparation, can solve the problems of not being effectively applicable to industrial production, complicated operation, insufficient yield, etc., and achieves low cost, simple synthesis process and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Synthesis of crude product of 2-(3-nitrobenzylidene) methoxyethyl acetoacetate
[0065] Add 2.4kg of methoxyethyl acetoacetate and 1.6kg of acetic anhydride to a 10L reactor, lower the internal temperature to 0-5°C, add 136g of concentrated sulfuric acid dropwise to control the internal temperature at 0-5°C, add 2.1kg of Nitrobenzaldehyde, the temperature was raised to 25-30°C for 6-7h, and the reaction was terminated. Add 95% ethanol, filter, and vacuum-dry at 45°C for 10 h to obtain a pale yellow solid.
[0066] (2) Refining of 2-(3-nitrobenzylidene) methoxyethyl acetoacetate
[0067] Put the crude product of methoxyethyl 2-(3-nitrobenzylidene)acetoacetate in a 30L reaction kettle, add 10kg of dichloromethane to dissolve it, then add saturated sodium bicarbonate solution and stir at room temperature for 30min, let stand and separate , adding pure water to the organic phase and stirring at room temperature for 30 min, standing to separate layers, and dichlorometh...
Embodiment 2
[0075] (1) Synthesis of crude product of 2-(3-nitrobenzylidene) methoxyethyl acetoacetate
[0076] Add 2.2kg of methoxyethyl acetoacetate and 1.3kg of acetic anhydride to a 10L reactor, lower the internal temperature to 0-5°C, add 136g of concentrated sulfuric acid dropwise under control of the internal temperature at 0-5°C, add 2.1kg of For nitrobenzaldehyde, heat up to 25-30°C for 2-3 hours to terminate the reaction. Add 95% ethanol, filter, and vacuum-dry at 45°C for 10 h to obtain a pale yellow solid.
[0077] (2) Refining of 2-(3-nitrobenzylidene) methoxyethyl acetoacetate
[0078] Put the crude product of methoxyethyl 2-(3-nitrobenzylidene)acetoacetate in a 30L reaction vessel, add 9.9kg of ethyl acetate to dissolve it, then add saturated sodium carbonate solution and stir at room temperature for 30min, let stand and separate , adding pure water to the organic phase and stirring at room temperature for 30 min, standing to separate layers, and distilling off the ethyl a...
Embodiment 3
[0086] (1) Synthesis of crude product of 2-(3-nitrobenzylidene) methoxyethyl acetoacetate
[0087] Add 3.3kg of methoxyethyl acetoacetate and 1.68kg of acetic anhydride to a 10L reactor, lower the internal temperature to 0-5°C, add 409g of concentrated sulfuric acid dropwise to control the internal temperature at 0-5°C, and add 2.1kg For nitrobenzaldehyde, the temperature was raised to 25-30°C for 11-12 hours, and the reaction was terminated. Add 95% ethanol, filter, and vacuum-dry at 45°C for 10 h to obtain a pale yellow solid.
[0088] (2) Refining of 2-(3-nitrobenzylidene) methoxyethyl acetoacetate
[0089] Put the crude product of methoxyethyl 2-(3-nitrobenzylidene)acetoacetate in a 30L reactor, add 9.6kg of chloroform to dissolve it, then add a saturated solution of potassium carbonate and stir at room temperature for 30min, let it stand for layers, organic Pure water was added to the mixture and stirred at room temperature for 30 min, and the layers were separated afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com