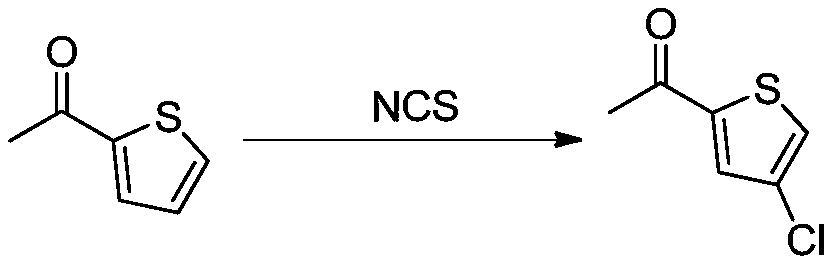
Method for synthesizing 2-acetyl-4-chlorothiophene
A technology of acetylthiophene and chlorothiophene, applied in directions such as organic chemistry, can solve the problems of high cost of trichloroisocyanuric acid, high content of dichloro by-products, unfavorable industrialized production, etc., and achieves environmental friendliness, less by-products, good quality The effect of market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, the method for synthesizing 2-acetyl-4-chlorothiophene of the present invention
[0029] Add 2-acetylthiophene (1kg, 7.925mol) and dichloromethane (10L) into the reaction flask, then add aluminum trichloride (3.17kg, 23.775mol) in batches, stir for 30min after the addition, and then Add N-chlorosuccinimide (NCS, 2.12kg, 15.85mol), and react at room temperature for 6 hours after the addition, and the reaction is complete as detected by TLC. Pour the reaction solution into 2L of cold water, separate the layers, extract the organic phase with water twice (500ml each time), and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness to obtain a crude product. Distillation under reduced pressure gave 914.0 g of 2-acetyl-4-chlorothiophene with a yield of 71.8%.
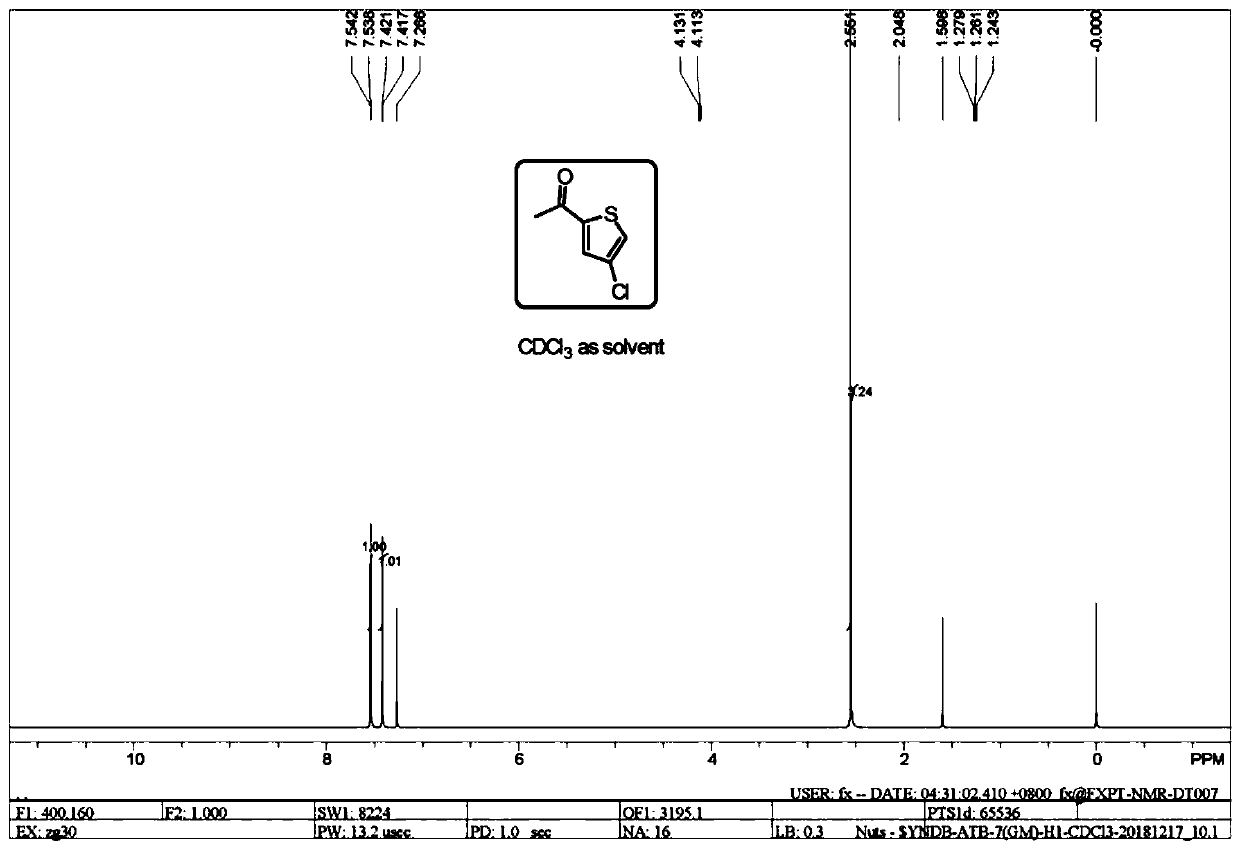
[0030] The HNMR spectrum of the 2-acetyl-4-chlorothiophene prepared by the present invention is as follows figure 1 shown. The HPLC collection of illustrative p...
Embodiment 2
[0031] Embodiment 2, the method for synthesizing 2-acetyl-4-chlorothiophene of the present invention
[0032] Add 2-acetylthiophene (1 kg, 7.925 mol) and dichloromethane (10 L) into the reaction flask, and then add zinc chloride (3.24 kg, 23.775 mol) in batches, and stir for 30 min after the addition is complete. Then N-chlorosuccinimide (NCS, 2.12 kg, 15.85 mol) was added in batches, and after the addition was completed, it was reacted at room temperature for 7 hours, and the reaction was complete as detected by TLC. Pour the reaction solution into 2L of cold water, separate the layers, extract the organic phase with water twice (500ml each time), and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness to obtain a crude product. Through distillation under reduced pressure, 869.4 g of 2-acetyl-4-chlorothiophene was obtained, with a yield of 68.3% and a purity of 99.58% upon detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com