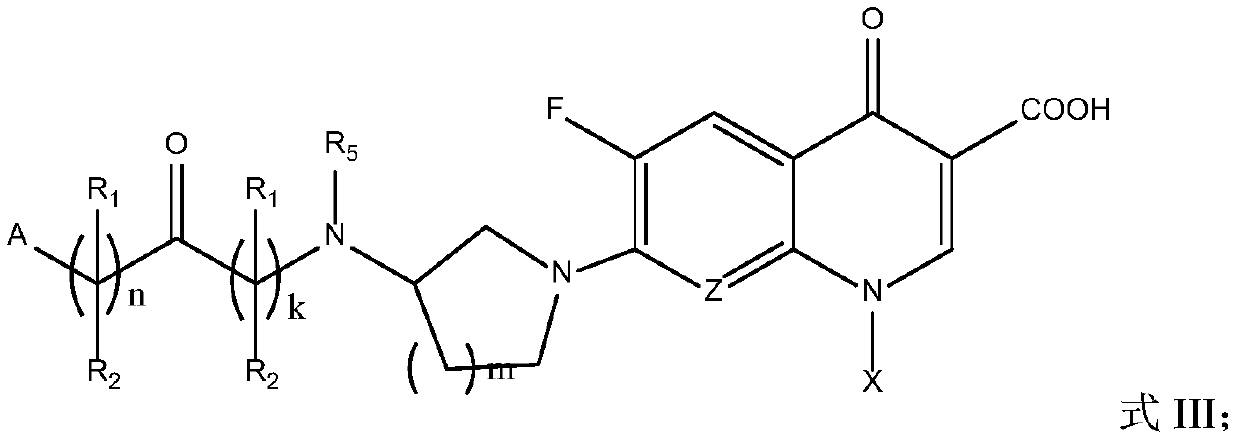
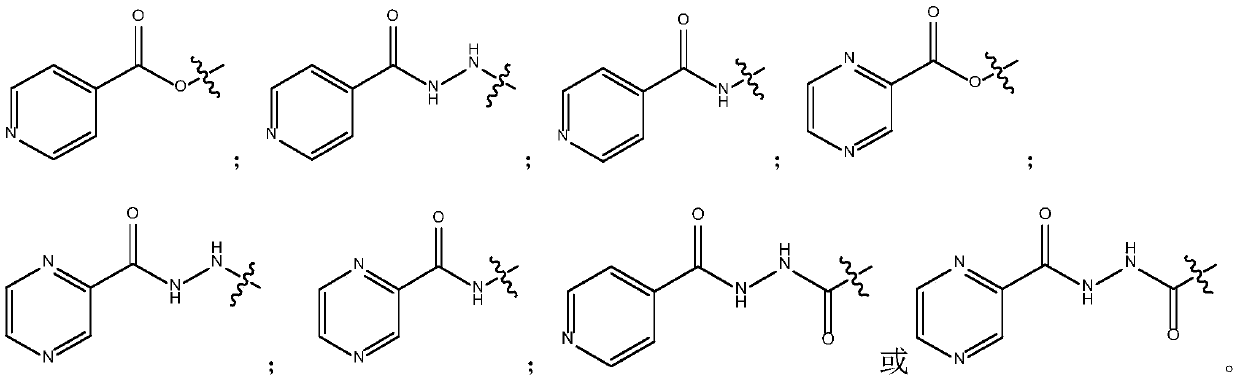
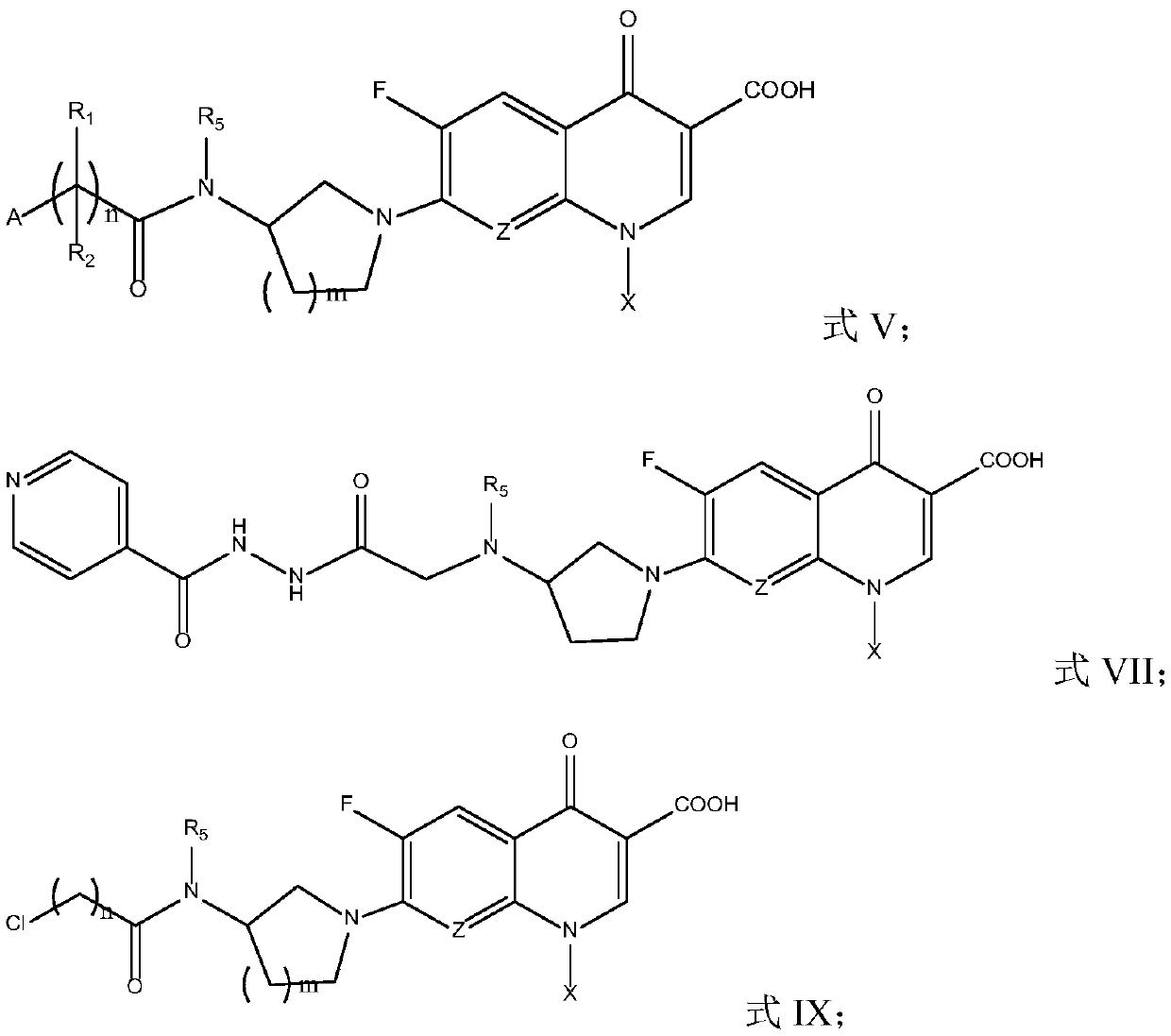
Fluoroquinolone amido derivatives and application thereof
An alkyl and hydroxyl technology, applied in the field of fluoroquinolone amine derivatives, can solve the problems of increased risk of drug compatibility, poor patient compliance, and increased difficulty in dose control, shortening the treatment cycle and reducing the dosage , reducing the effect of the type of drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1-24
[0079] Add 1mmol Floxacin and 2mL DCM to a 100mL round bottom flask, cool in an ice bath, stir magnetically, add 3mmolNaHCO 3 After 20min, 2.5mmol chloroacyl chloride (Cl-(CH 2 ) n COCl, n=1, 2, 3) in DCM (2mL) solution (the drop rate is about 1d / 2s), the reaction continued under the ice bath (at 2-CC, moved to -5°C) after dropping, and TLC tracked and monitored to The reaction is over. Stirring was stopped and H was added 2 O 15mL and DCM 20mL, under stirring, use 1N HCl solution to adjust pH=3-4, if there is solid, then stand still, filter with suction, wash the filter cake 3 times with DCM, and keep the filter cake for further purification; the filtrate is transferred to a separatory funnel, The liquid was separated, the aqueous phase was extracted with DCM (15 mL×1), the organic phases were combined, washed with saturated NaCl solution (15 mL×1), the organic phase was collected, anhydrous NaCl 2 SO 4 dry. The crude product was spin-dried by a rotary evaporator, and t...
Embodiment 1-35
[0085] In the 100mL round bottom flask, add 1mmol of the intermediate of formula X, 5mL of toluene, 2mmol of Et 3 N, stir for 20-30min, add 2mmol isonicotinic acid (INA) or pyrazinecarboxylic acid (POA), after 10min, move to 110℃~120℃ oil bath for reflux reaction, monitor by TLC until the reaction is complete. Spin the reaction solution to dryness with a rotary evaporator, add 30mL DCM, stir to dissolve, filter with suction, wash the filter cake with DCM for 3 times, and wash the filtrate with 10% citric acid solution (15mL×1), collect the organic phase, anhydrous Na 2 SO 4 dry. The crude product was spin-dried by a rotary evaporator, the pure product was obtained by column chromatography, and the target product was obtained by vacuum drying.
[0086] Table 2 target compound formula XI synthetic result
[0087]
[0088]
[0089]
[0090] Table 3 target compound formula XI synthetic result
[0091]
[0092]
[0093]
[0094] Synthesis of formula XII serie...
Embodiment 36-41
[0097] Add 2mmol isoniazid, 4mmol NaHCO 3 and 5mL DCM, stirred at -5°C for 20min, then added dropwise 3mmol of chloroacetyl chloride solution (dropping rate is about 1d / 2s) with a constant pressure dropping funnel, and continued to react at -5°C, followed by TLC monitoring until the end of the reaction . While stirring, add DCM-CH 3 OH solution (V DCM : V CH3OH =2:1) until the solids in the reaction flask no longer decrease, let stand, filter with suction, and use DCM-CH for the filter cake 3 OH solution was washed 3 times, and the filtrate was washed with anhydrous Na 2 SO 4 Dry and spin dry with a rotary evaporator to obtain a crude product, then add 5 mL of DCM and stir for 20 min, filter with suction, wash the filter cake 3 times with DCM, and dry in vacuo to obtain an intermediate for future use.
[0098] Add 1mmol of Floxacin and 4mmol of Et in a 100mL round bottom flask 3 N and 2mL DMF, after stirring for 20min, add the above-mentioned intermediate, and after 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com