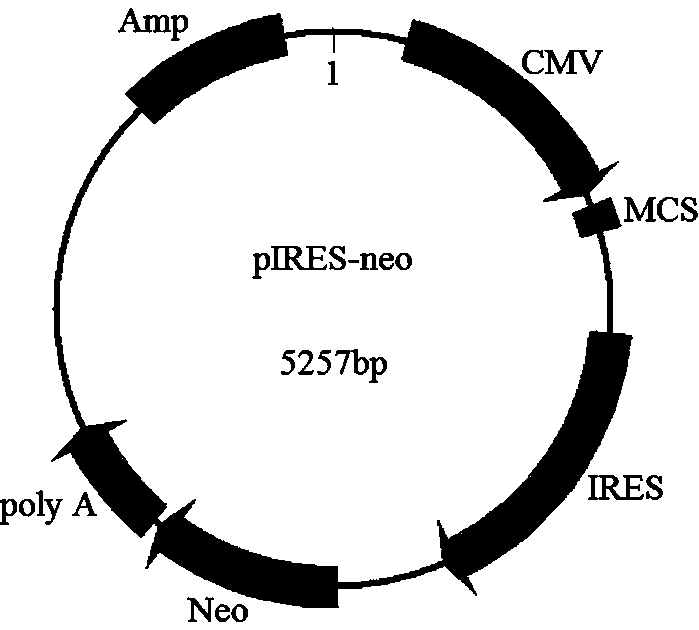
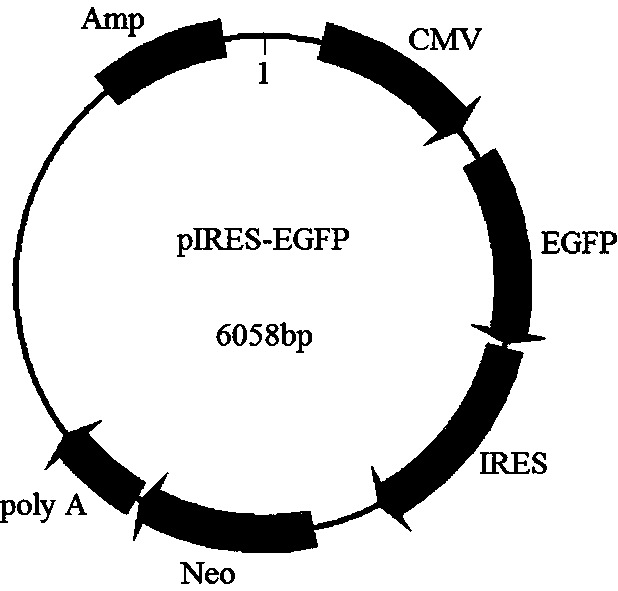
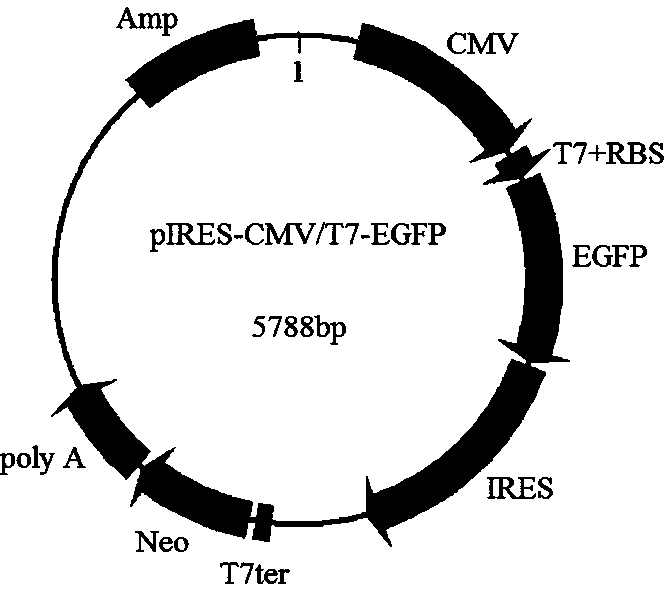
Double-promoter expression vector and construction method thereof
A dual promoter and expression vector technology, applied in the field of genetic engineering, can solve the problems of high cost and cumbersome operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In this example, the EGFP gene is used as the target gene and pIRES-neo is used as the starting vector as an example, and the construction process of the dual expression vector pIRES-CMV / T7-EGFP described in this application is introduced as follows.
[0052] (1) For the target gene EGFP, design primers and perform PCR amplification
[0053] Using the EGFP (Enhanced green fluorescent protein) gene (GenBank: U55763.1, 613-1329 bases) sequence in the pEGFP-C1 plasmid as the target gene, design primers P1 and P2 as follows:
[0054] P1: 5′-CCGGAATTCATGGTGAGCAAGGGCGAGGAG-3′, (the partial sequence of GAATTC at the 5′ end is the introduced Eco RI restriction site)
[0055] P2: 5′-CTAGGATCCCTTGTACAGCTCGTCCATGCCGA-3′; (the partial sequence of GGATCC at the 5′ end is the introduced BamHI restriction site)
[0056] The pEGFP-C1 plasmid was used as a template for PCR amplification (the size of the amplified product was 717 bp). After electrophoresis detection, the PCR amplificati...
Embodiment 2
[0091] In order to determine the expression of the target gene EGFP in eukaryotic cells or prokaryotic cells, the dual promoter expression vector pIRES-CMV / T7-EGFP recombinant plasmid constructed in Example 1 was further transfected into CHO-S cells and Transformation of Escherichia coli BL21, the specific experimental process is as follows.
[0092] (1) Expression vector transfection CHO cell expression system
[0093] CHO-S cells were pre-cultured in DMEM-F12 medium containing 10% fetal bovine serum and 1% penicillin / streptomycin, and the culture conditions were: 37°C, 5% CO 2 Cultured in an incubator, when the cell adherent growth density reached 90%, digested with 0.25% trypsin and collected the cells, 2 × 10 5 Seed each well in a 24-well plate, and use Lipo2000 (Lipofectamine® 2000) as the transfection reagent for transfection when the cell density reaches (about 24 hours) 60%-70%.
[0094] During the transfection process, the pIRES-EGFP vector (constructed in step (2) ...
Embodiment 3
[0108] On the basis of Example 2, in order to further investigate whether the dual-promoter vector constructed in this application is suitable for the expression of other target proteins, the inventor further used ASFVp54 as the target protein, taking this as an example to construct a recombinant dual-promoter vector pIRES- CMV / T7-p54 was translated and expressed using eukaryotic expression system and prokaryotic expression system respectively. The specific process is as follows.
[0109] The gene sequence (576bp) corresponding to the target protein ASFVp54 is as follows:
[0110] ATGGATTCTGAATTTTTTCAACCGGTTTATCCGCGGCATTATGGTGAGTGTTTGTCACCAGTCTCTACACCAAGCTTCTTCTCCACACATATGTATACTATTCTCATTGCTATCGTGGTCTTAGTCATTATTATCATCGTTCTAATTTATCTATTTTCTTCAAGAAAGAAAAAAGCTGCTGCCGCTATTGAAGAGGAAGATATACAGTTTATAAATCCTTATCAAGATCAGCAGTGGGTAGAGGTCACTCCACAACCAGGTACCTCTAAACCGGCTGGAGTGACTACAGCAAGTGTAGGCAAGCCAGTCACGGGCAGGCCGGCAACAAACAGACCAGTTACGGACAGGCTAGTCATGGTAACTGGCGGGCCAGCGGCCGCAAGTGCGGCCGCAAGTGCGGCTGC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com