Method for detecting parecoxib sodium genotoxic impurities
A technology of parecoxib sodium and genotoxicity, which is applied in the field of detection of genotoxic impurities of parecoxib sodium, can solve the problems of unsatisfactory and high detection limit, and achieve simple operation, high specificity and durability, The effect of high detection precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The elution condition of embodiment 1 is:
[0066] Keep the volume fraction of 0.5% acetic acid aqueous solution at 80%, and keep the volume fraction of acetonitrile at 20%.
Embodiment 2
[0067] The elution condition of embodiment 2 is:
[0068] Within 0min-4.0min, the volume fraction of mobile phase A decreased from 80% to 20%, and the volume fraction of acetonitrile increased from 20% to 80%.
Embodiment 3
[0069] The elution condition of embodiment 3 is:
[0070] 0min-6.0min, the volume fraction of mobile phase A decreased from 80% to 20%, and the volume fraction of acetonitrile increased from 20% to 80%.
[0071] The elution condition of embodiment 4-8 is:
[0072] 0min-3.0min, the volume fraction of mobile phase A drops to 20% by 80%, and the volume fraction of acetonitrile rises to 80% by 20%.
[0073] Draw the following conclusions by the mass spectrogram of embodiment 1-8,
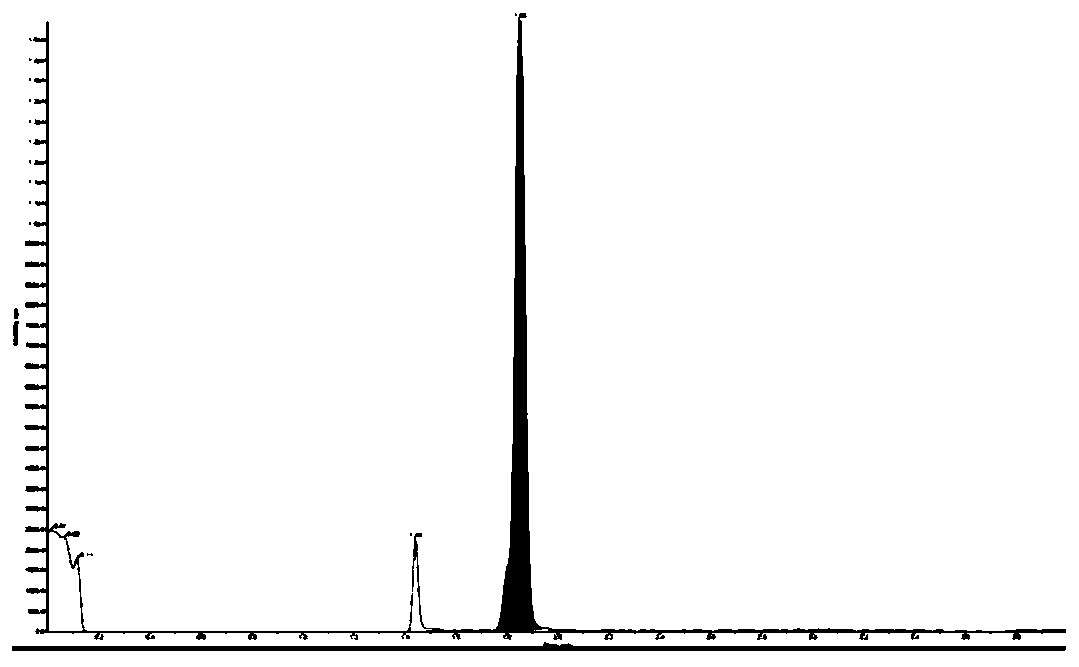
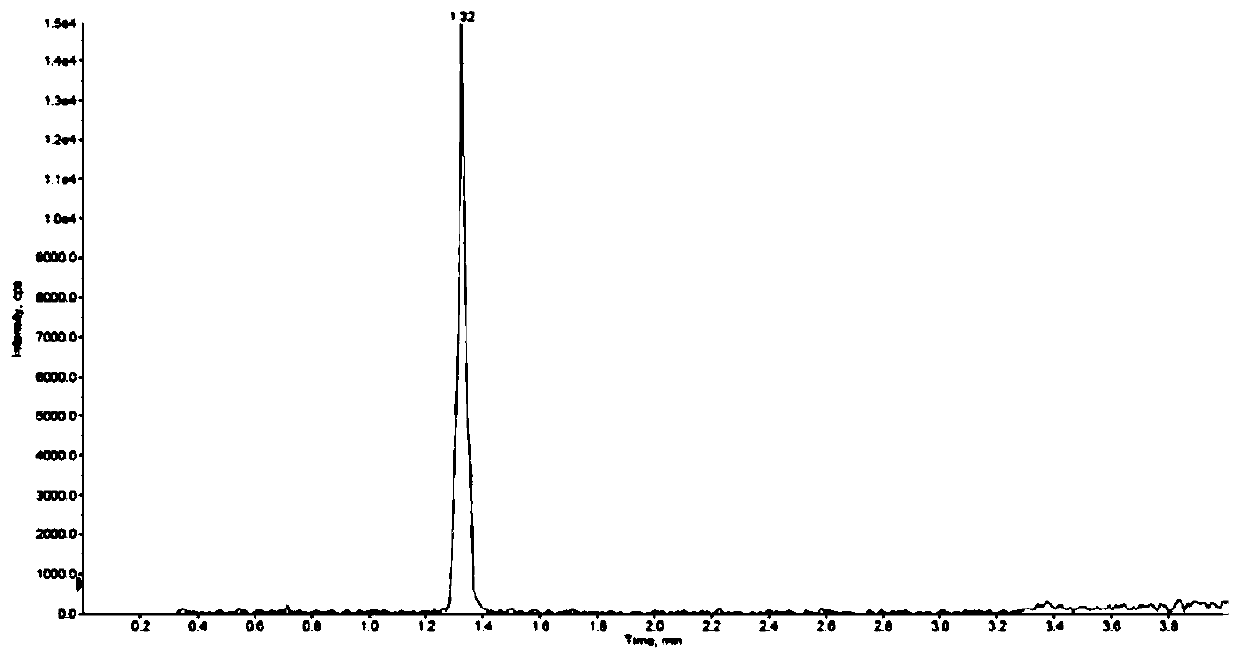
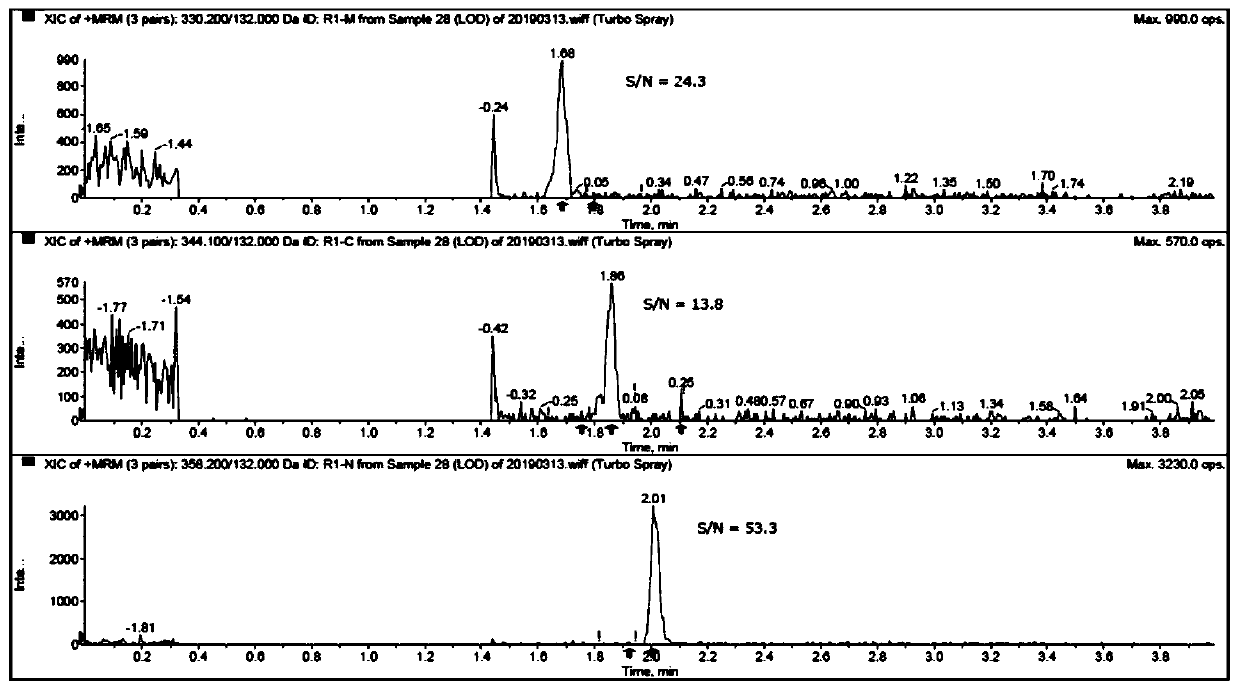
[0074] In Example 1, the main components and impurities were all peaked out within 8 minutes, and the separation between impurities was good, and the impurities R1-M could not be completely separated from the main components.
[0075] In embodiment 2, main component and impurity all go out peak and complete within 8 minutes, and each component peak shape is good, and main component and each impurity baseline are separated, and the degree of separation between impurities reaches 1.3. The mass spectrum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com