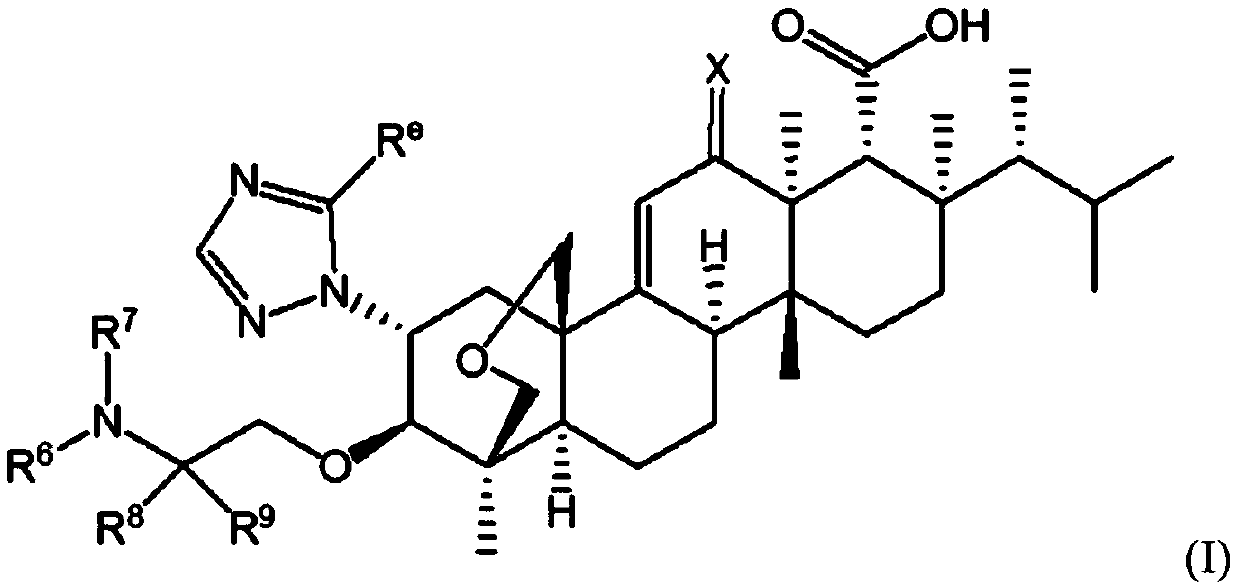
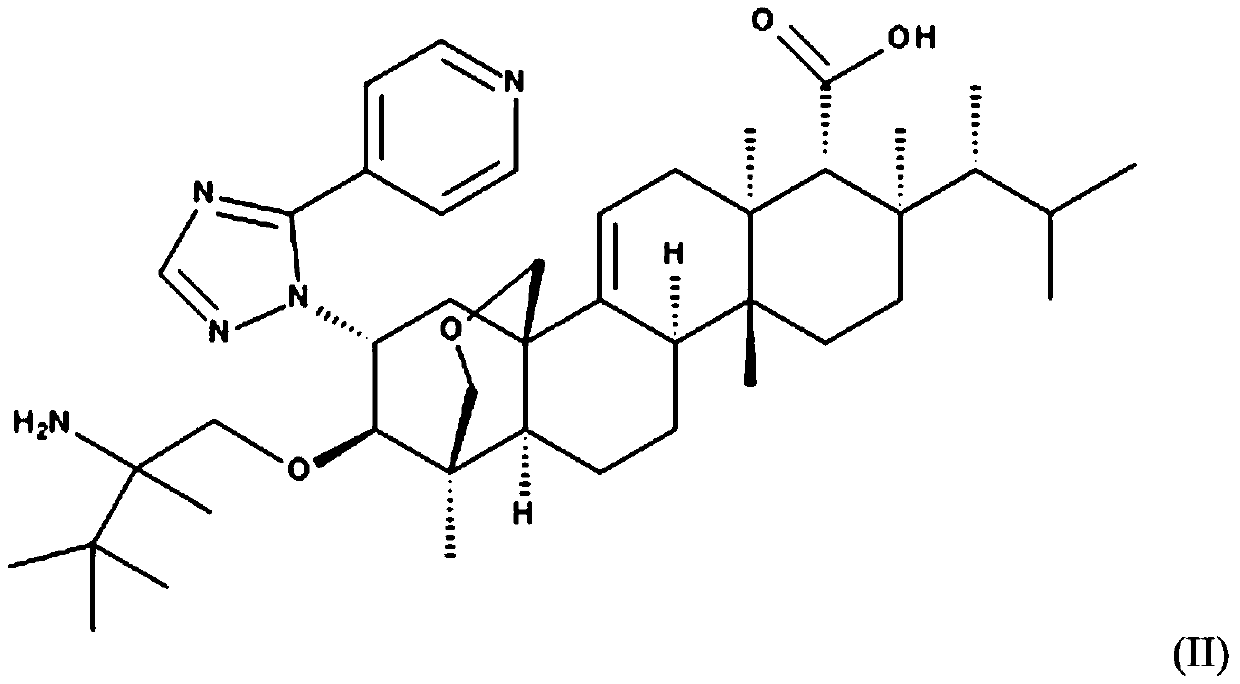
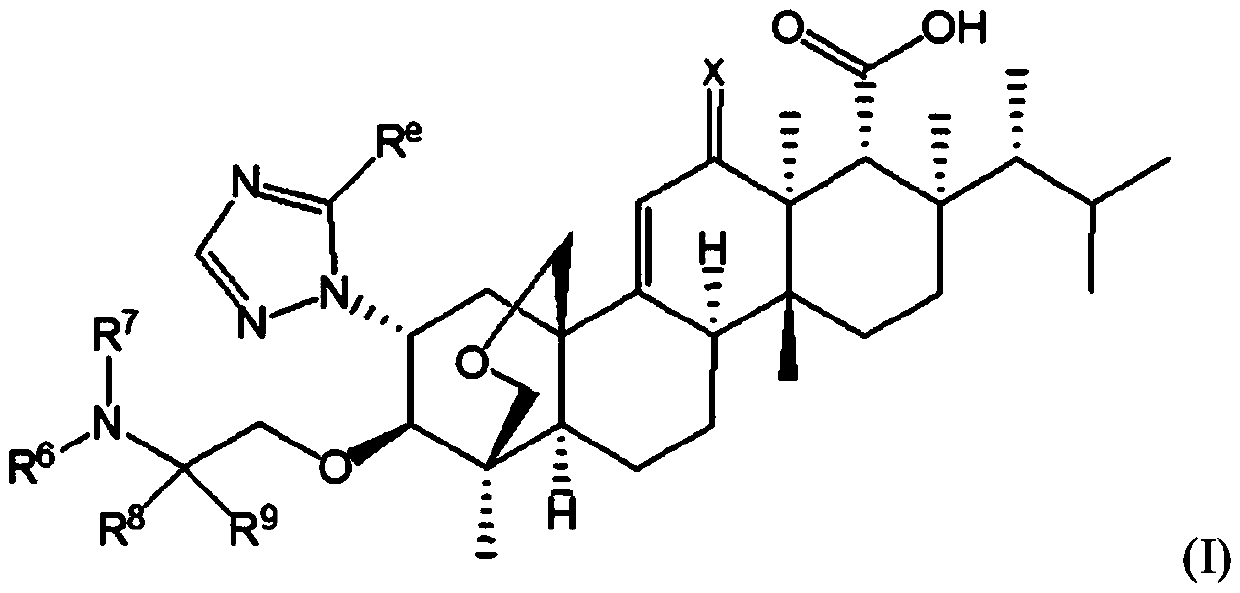
Antifungal agents with enhanced activity in acidic ph
A fungal infection and mold technology, applied in the direction of antifungal agents, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of anti-candida antifungal performance reduction and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0125] Evaluation of the effect of pH on the susceptibility of clinical vaginal Candida glabrata and Candida albicans isolates
[0126] The purpose of this study was to determine whether changes in the pH of the test medium were beneficial to representative compounds of Candida glabrata and Candida albicans vaginal isolates to enfufungin derivatives using fluconazole and micafungin as comparators (e.g. Citrate of SCY-078) has an effect on the in vitro susceptibility.
[0127] Materials and methods
[0128] Clinical isolates and antifungal agents
[0129] Ten strains each of C. glabrata and C. albicans vaginal isolates were tested. Strains were obtained from recent clinical trial patients with vulvovaginal candidiasis (VVC) prior to treatment. Candida strains were obtained from the Mycology Reference Library (MRL) at Case Western Reserve University School of Medicine, Ohio, USA.
[0130] Antifungal Susceptibility Testing
[0131] Susceptibility testing was performed us...
example 2
[0175] Vaginal concentrations of SCY-078 after oral administration to mice
[0176] The goal of this study was to determine the exposure of SCY-078 in vaginal tissue and secretions after oral administration of SCY-078 to mice and the relationship between this exposure and the concentration of SCY-078 in plasma, that is, the experimental parameters of Candida infection. Identify the model.
[0177] method
[0178] Female CD-1 mice were administered SCY-078 by oral gavage (n=3 / time point / dose group) for one, two or eight doses ranging from 10 to 80 mg / kg QD (once daily ) and BID (twice daily) dosage regimen with and without the following starting doses:
[0179] QD (Day 1): 10, 20, 40, 80mg / kg
[0180] BID (Day 1): 10 / 5, 20 / 10, 40 / 20, 80 / 40mg / kg
[0181] BID repeat dose (Day 1 to Day 4): 10 / 5, 20 / 10 40 / 20, 80 / 40 mg / kg on Day 1; 5, 20, 20, 40mg / kg BID
[0182] Blood, vulvovaginal tissue, and vaginal secretions were collected prior to dosing and at 1, 2, 4, 6, 8, 12, 18, and...
example 3
[0191] A Phase 2 Study of SCY-078 in Moderate and Severe Vulvovaginal Candidiasis (VVC)
[0192] This study is a proof-of-concept study designed to evaluate the safety and efficacy of two dosing regimens of oral SCY-078 (administered as phosphate) in individuals presenting with moderate to severe VVC.
[0193] method
[0194] Key selection criteria include:
[0195] 1. Individuals with moderate to severe VVC as determined from a vaginal discharge sample by a positive potassium hydroxide (KOH) test
[0196] 2. Three episodes of vaginitis in the last year determined to be caused by Candida or responding to antifungal therapy
[0197] Subjects were randomized in a 1:1:1 ratio to one of three treatment groups: oral SCY-078 at a starting dose of 1250 mg SCY-078 followed by 750 mg SCY-078 QD for 2 or 4 days, or oral fluoride Conazole 150mg for 1 day.
[0198] Subjects were assessed on Day 24 (Test of Cure Visit), Day 60, Day 90, and Day 120 (End of Study).
[0199] Analysis inclu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com