Rhuschalcone compounds and application thereof
A bis-chalcone and compound technology, applied in the application field of this type of compound, can solve the problems that the research results have not been paid attention to, and achieve the effect of good antifungal effect and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
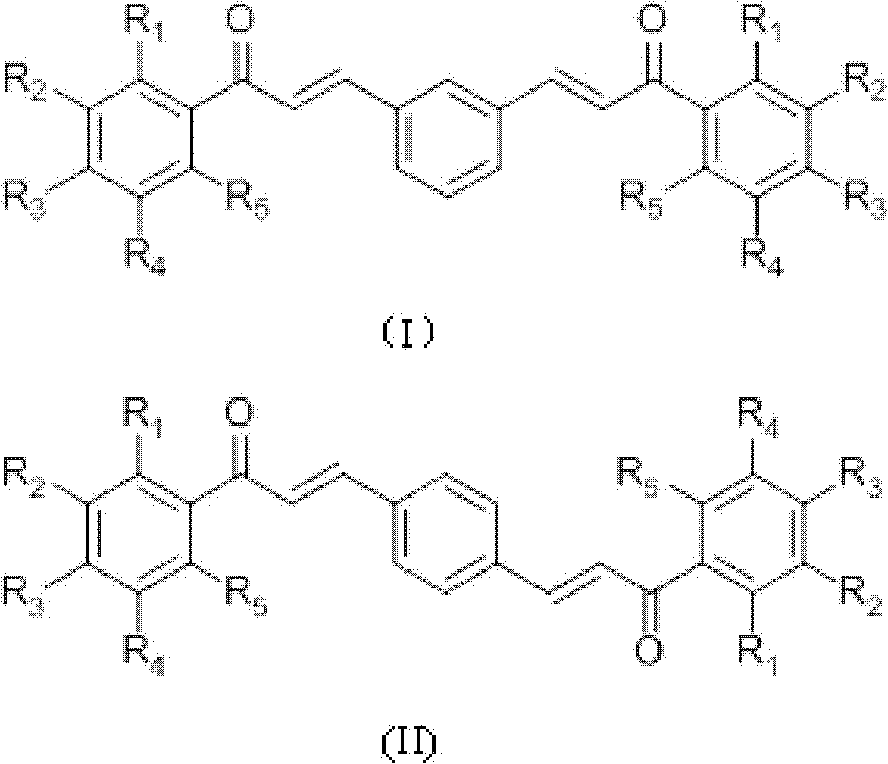
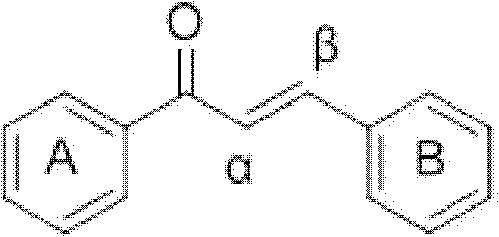
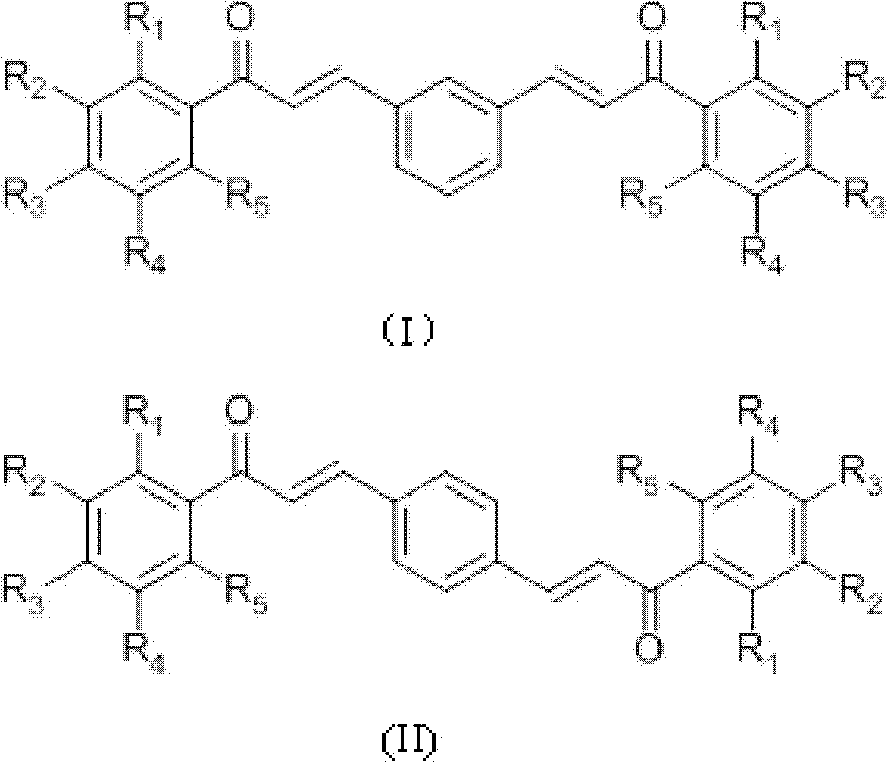
[0035] Preparation of compound 1-6 (see Table 1 for specific structural formula): acetophenone derivative (0.02mol), m-phthalaldehyde or terephthalaldehyde (0.01mol), absolute ethanol 18mL, thionyl chloride 1.8mL Add it into a 100mL three-neck flask, react at room temperature for 2-8 hours, monitor the reaction by TLC (thin layer chromatography), stop the reaction until the reaction is complete, and add 10mL distilled water to aid precipitation. Vacuum filter, wash with a large amount of cold distilled water, then wash the product once with 100 mL of ethanol at 65°C to remove impurities, filter while hot, and dry in vacuum at 60°C to obtain the product.
[0036] m-Hydroxyacetophenone and m-phthalaldehyde prepare 3'-hydroxyl-3-(3-(3-hydroxyphenyl)-3-carbonyl-propenyl)chalcone (compound 1) according to Example 1, yield : 42.54%, Mp: 239.7-241.9°C. 1 HNMR (CD 3 COCD3, δ, ppm): 7.130, 7.136, 7.150, 7.156 (dd, 2H, J = 8.0, 2.4Hz, 4, 6-H), 7.389, 7.409, 7.429 (6.923, 6.945 (d, 4H,...
Embodiment 2
[0043] Preparation of Compound 7-11 (see Table 1 for specific structural formula): Acetophenone derivatives (0.01mol), terephthalaldehyde or m-phthalaldehyde (0.005mol), absolute ethanol 20mL, 10mL 10% NaOH ( W / V) into a 100mL three-neck flask, react at room temperature for 1-5 days, add 50mL of distilled water, neutralize with 3M HCl to pH = 6, filter under reduced pressure, dry the filter residue, use silica gel column chromatography, and recrystallize. Dry to obtain the product.
[0044] 2-Hydroxyl-6-methoxymethoxyacetophenone and terephthalaldehyde prepare 2'-hydroxyl-6'-methoxymethoxyl-4-(3-(2-hydroxyl-6- Methoxymethoxyphenyl)-3-carbonyl-propenyl)chalcone (compound 7), yield 14.01%. MP: 190.4-192.0°C. 1 H NMR (CDCl 3 , δ, ppm): 3.541 (s, 6H, 6×H-CH 3 ), 5.323(s, 4H, 4×H-CH 2 ), 6.605, 6.625 (d, 2H, J=8.0, 2×3′-H), 6.672, 6.674, 6.693, 6.695 (dd, 2H, J=8.2Hz, 2×5′-H), 7.343, 7.364 , 7.385(t, 2H, J=8.4Hz, 2×4′-H), 7.662(s, 4H, 1×2, 3, 5, 6-H), 7.789, 7.828(d, J=15.6Hz...
Embodiment 3
[0054] Embodiment 3 antibacterial activity
[0055] After the test bacteria were taken out from the refrigerator at 4°C, they were inoculated on the medium of the petri dish: the bacteria were inoculated on the beef extract peptone medium, and cultured at 37°C for 24 hours; the mold was inoculated on the PDA medium, and cultivated at 28°C for 48 hours. stand-by. Then, the 13 compounds synthesized were made into 5 gradient concentrations (2500 μg / mL, 1000 μg / mL, 500 mg / mL, 250 μg / mL, 100 μg / mL) with ethyl acetate, and were added to each petri dish by casting method. 9mL of sterilized culture medium and 1mL of sample were used, and the control group was replaced by 1mL of ethyl acetate and sterile water, and the other was the same. The medium was solidified and then inoculated with a 6mm hole punch. Then put them into a constant temperature and humidity incubator for cultivation, with bacteria at 37°C and fungi at 28°C. When the colony on the control group was about to cover ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com