Method for synthesizing 2-cyano-5-aryl-1H-imidazole compound
A compound and imidazole technology, applied in the field of pesticide technical and intermediate synthesis, can solve the problems of complex treatment process, harsh reaction conditions, and many waste water, etc., and achieve the effects of high yield and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
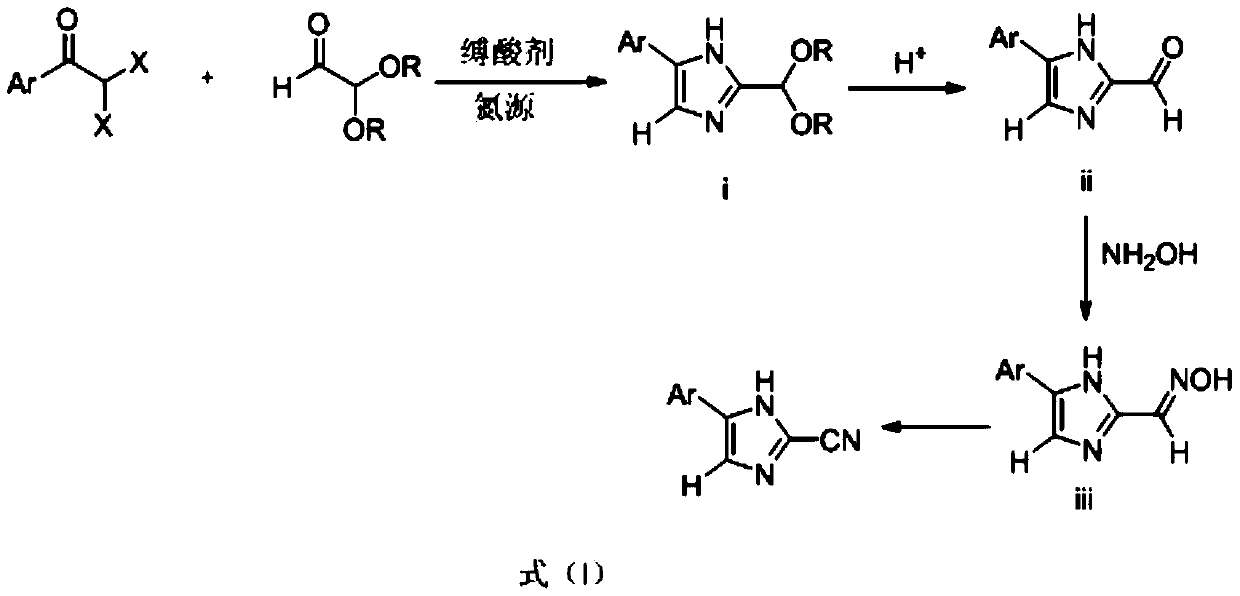
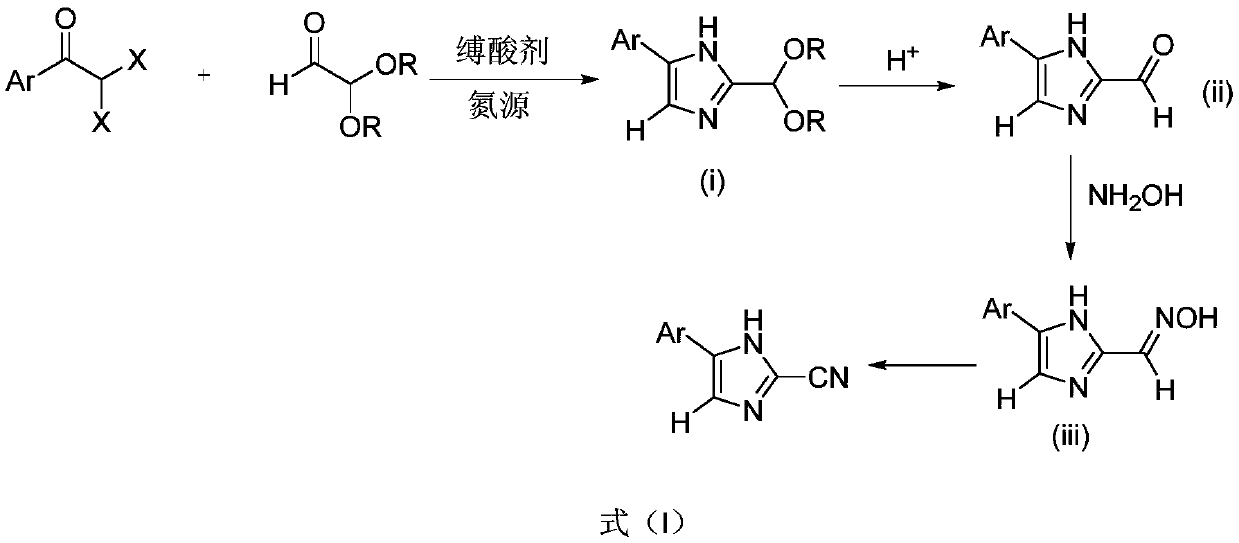
[0024] 27.8g of α,α-dibromophenylethanone, 139g of methanol, 34.7g of 60% aqueous solution of 2,2-dimethoxyacetaldehyde, 30.4g of triethylamine, and 29.6g of ammonium acetate were added with a thermometer, refluxing In a three-necked round-bottomed flask of a condenser and a stirring device; heat up to 40°C under stirring, stir the reaction, monitor the reaction by TLC, the raw material α,α-dibromophenylethanone disappears to complete the reaction (about 10h); add water at 40°C 139g was stirred for 1h, a large amount of solid was formed, and the crude product was obtained by filtration; the above-mentioned filtered solid was beaten with 50g of warm water at 40°C, stirred for 1h, filtered, and dried to obtain the product 2-(dimethoxymethyl)-5-phenyl-1H-imidazole 19.2g, yield 91%, content 96.3%.
Embodiment 2
[0026] 18.9g of α,α-dichlorophenylethanone, 139g of methanol, 34.7g of 60% aqueous solution of 2,2-dimethoxyacetaldehyde, 30.4g of triethylamine, and 29.6g of ammonium acetate were added in sequence with a thermometer, refluxing In the three-necked round-bottomed flask of the condenser and stirring device; heat up to 40°C under stirring, stir the reaction, monitor the reaction by TLC, the raw material α,α-dichlorophenylethanone disappears to complete the reaction (about 16h); add water at 40°C 139g was stirred for 1h, a large amount of solid was formed, and the crude product was obtained by filtration; the above-mentioned filtered solid was beaten with 50g of warm water at 40°C, stirred for 1h, filtered, and dried to obtain the product 2-(dimethoxymethyl)-5-phenyl-1H-imidazole 18.4g, yield 84.3%, content 95.8%.
Embodiment 3
[0028] 27.8g of α,α-dibromophenylethanone, 139g of methanol, 34.7g of 60% aqueous solution of 2,2-dimethoxyacetaldehyde, 30.4g of triethylamine, and 19.2g of ammonium carbonate were added in sequence with a thermometer, refluxing In a three-necked round-bottomed flask of a condenser and a stirring device; heat up to 40°C under stirring, stir the reaction, monitor the reaction by TLC, the raw material α,α-dibromophenylethanone disappears to complete the reaction (about 10h); add water at 40°C 139g was stirred for 1h, a large amount of solid was formed, and the crude product was obtained by filtration; the above-mentioned filtered solid was beaten with 50g of warm water at 40°C, stirred for 1h, filtered, and dried to obtain the product 2-(dimethoxymethyl)-5-phenyl-1H-imidazole 19.7g, yield 90.2%, content 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com