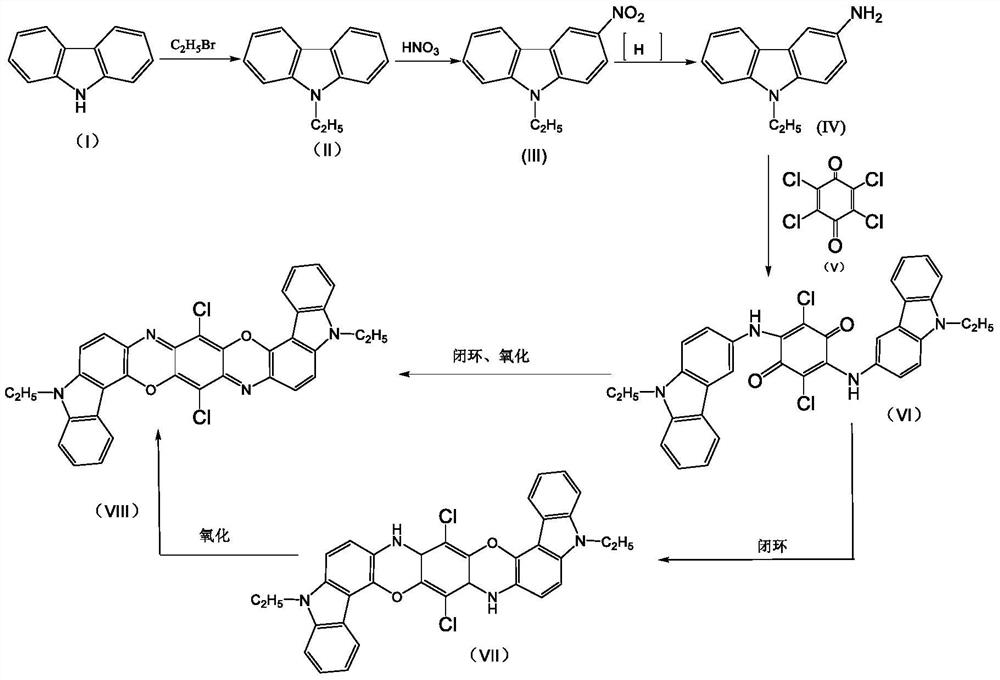
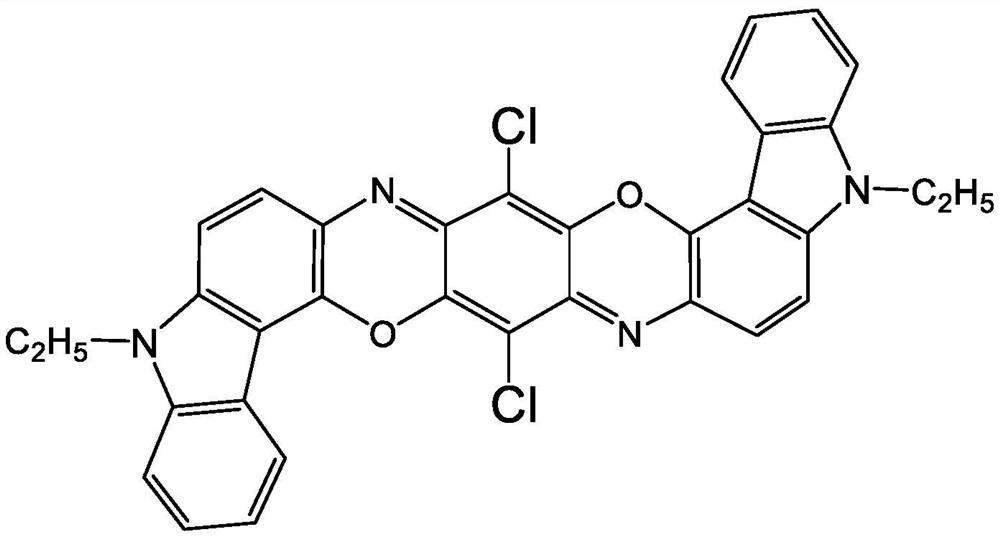
A kind of preparation method of pigment violet 23 crude product
A technology for pigment purple and crude products, applied in the direction of bisazine oxazine dyes, etc., can solve problems such as environmental pollution and complicated processes, and achieve the effect of improving purity, quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The present invention provides a kind of preparation method of Pigment Violet 23 crude product, comprises the following steps:
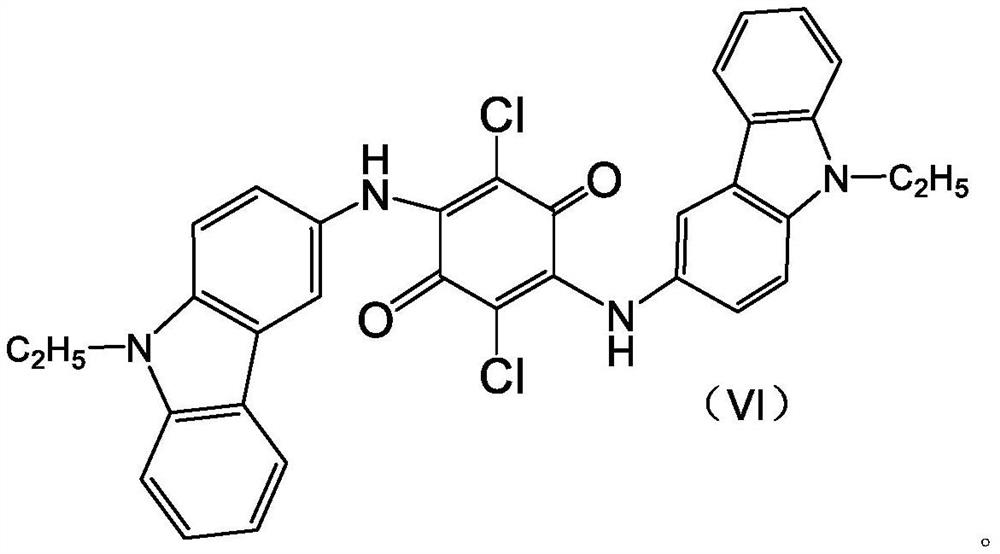
[0026] Step 1, add a certain amount of heteropolyacid to the organic solution containing condensate 2,5-dichloro-3,6-bis(9-ethyl-3-carbazole amino)-1,4-benzoquinone as The ring-closing oxidation catalyst is reacted at 125-145° C. under normal pressure for a period of time, wherein the amount of the heteropolyacid added is 0.1-5.0% of the mass of the condensate.
[0027] The molecular formula of the condensate 2,5-dichloro-3,6-bis(9-ethyl-3-carbazolylamino)-1,4-benzoquinone is as follows:
[0028]
[0029] In this embodiment, the method for preparing the condensate 2,5-dichloro-3,6-bis(9-ethyl-3-carbazolylamino)-1,4-benzoquinone can adopt any one of the prior art made by a process. For example:
[0030] The preparation process of the 2,5-dichloro-3,6-bis(9-ethyl-3-carbazole amino)-1,4-benzoquinone condensate is as follows:
[0031] 3-ami...
Embodiment 1
[0049] To 200g condensate 2,5-dichloro-3,6-bis(9-ethyl-3-carbazolamino)-1,4-benzoquinone and o-dichlorobenzene solvent mixture (containing 6.1% condensation Add phosphomolybdic acid [H 3 P(M O3 o 10 ) 4 ·XH 2 O, CAS No.51429-74-4] 0.122g, keep warm in a three-neck flask at 140°C, reflux at normal pressure for 4 hours, filter, wash with ethanol first, then wash with hot water, and dry to obtain 11.25g of Pigment Violet 23 The crude product was dissolved in pyridine, filtered and washed to remove impurities, and 11.02 g of pure crude Pigment Violet 23 was obtained, with a calculated purity of about 98% and a yield of 92.7%.
[0050] Mass spectrometry data proved that the molecular weight of the product was consistent with the theoretical value. MALDI-TOF (DHBA, +H): 588, theoretical value 588.11.
Embodiment 2
[0052] To 200g 2,5-dichloro-3,6-bis(9-ethyl-3-carbazolamino)-1,4-benzoquinone and o-dichlorobenzene solvent mixture (containing 6.1% condensate) Add phosphotungstic acid equivalent to 0.5% weight of condensate [H 3 PO 4 (WO 3 )·X H 2 O, CASNo.12067-99-1] 0.061g, keep warm in a three-necked flask at 140°C, reflux at normal pressure for 4 hours, filter, wash with ethanol first, then wash with hot water, and dry to obtain 11.61g of crude pigment violet 23 , dissolved in pyridine, filtered and washed to remove impurities to obtain 11.49 g of pure crude Pigment Violet 23, with a calculated purity of about 99% and a yield of 95.6%.
[0053] Mass spectrometry data proved that the molecular weight of the product was consistent with the theoretical value. MALDI-TOF (DHBA, +H): 588, theoretical value 588.11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com