AAV double-vector gene treatment system and application thereof to treatment of type II MPS
A gene therapy and vector technology, applied in the field of medical technology treatment, can solve problems such as poor treatment effect, and achieve the effects of enhancing safety, shortening time consumption, and improving the expression of IDS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 Construction of adeno-associated virus dual vector gene therapy system
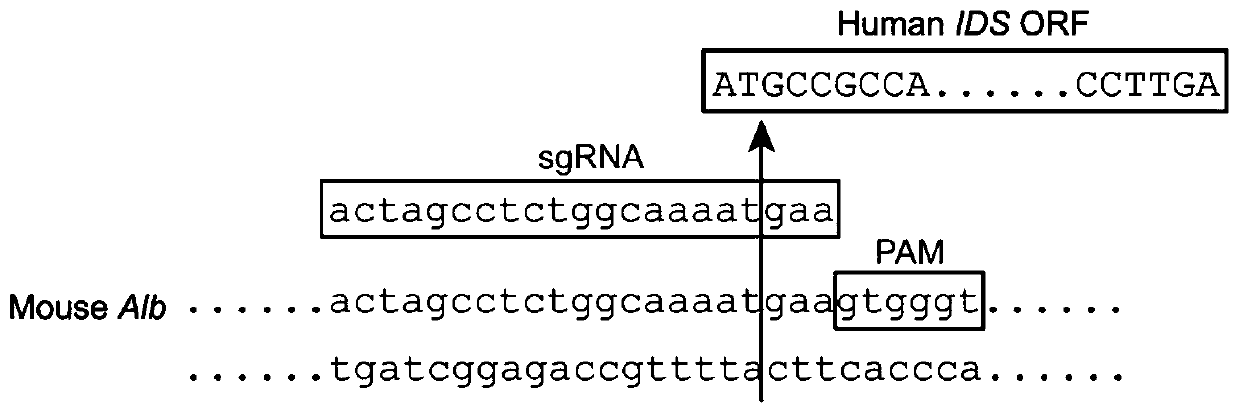
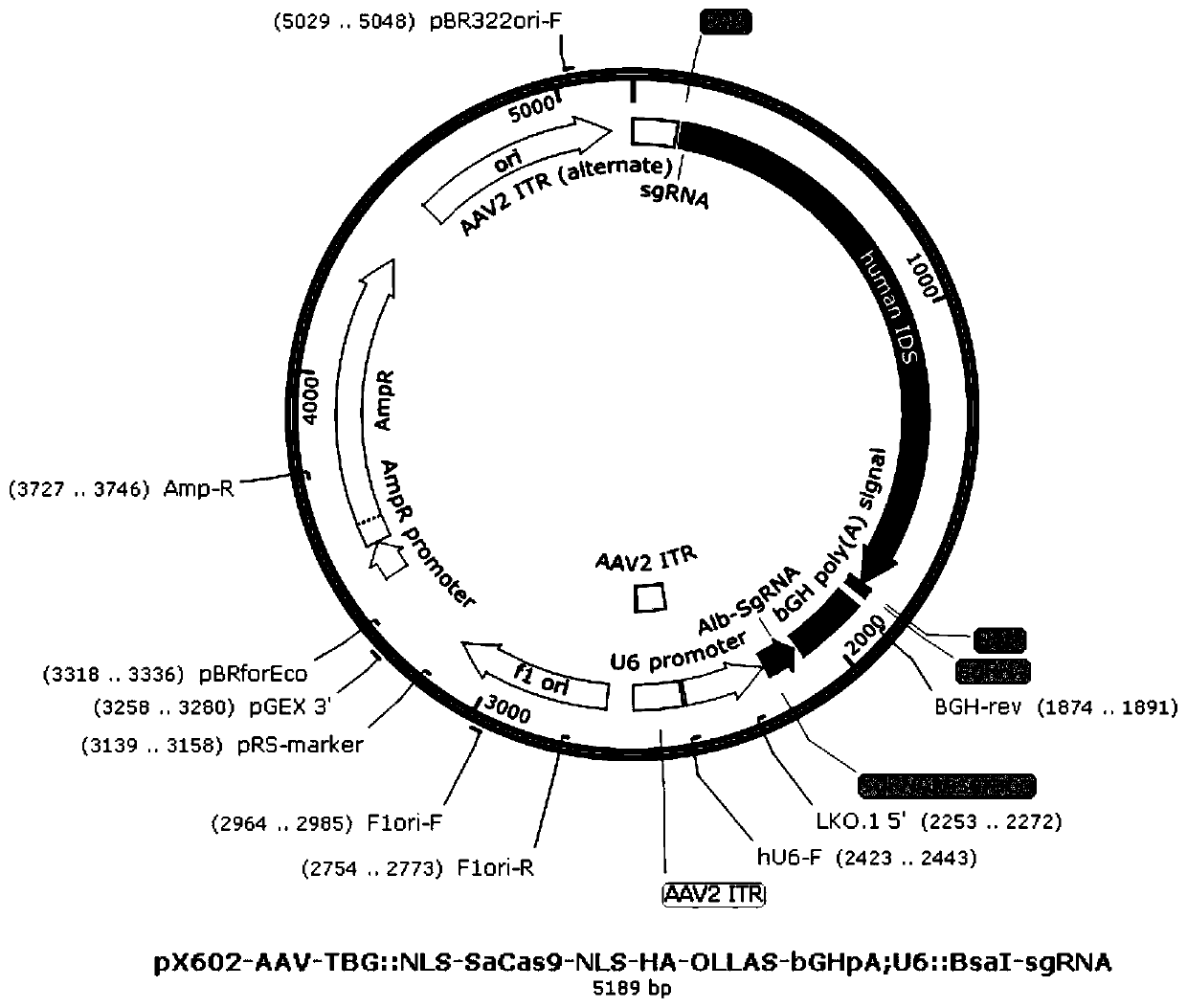
[0034] An adeno-associated virus dual-vector gene therapy system for mucopolysaccharidosis type II gene therapy consists of a first vector and a second vector, wherein the first vector is designed to carry the targeted mouse albumin gene (Albumin , Alb) cleavage site-specific small guide RNA (small guide RNA, sgRNA) and the open reading frame (ORF) of the human iduronate-2-sulfatase (human iduronate-2-sulfatase, hIDS) gene ) sequence (NM_000202.8), the mouse albumin gene was analyzed and evaluated through the sgRNA design website (URL: https: / / portals.broadinstitute.org / gpp / public / analysis-tools / sgrna- design) to design sgRNA against mouse albumin gene (sequence: 5'ACTAGCCCTCTGGCAAAATGAA3'). The AAV-DJ vector targets the liver cells of mice with mucopolysaccharidosis Ⅱ disease, and the sgRNA recognizes a specific part of the albumin gene (Alb) in the mouse liver cells (the cleavage si...
Embodiment 2
[0037] Example 2 Gene therapy experiment of adeno-associated virus dual vector gene therapy system on MPSII disease mouse model
[0038] MPSII disease mouse model (B6N.Cg-Ids tm1Muen / J, item number: 024744) were purchased from The Jackson Laboratory (USA), and a total of 2 male breeding mice (Ids X+ / Y ) and 3 Ids gene heterozygous female breed mice (Ids X+ / X- ). Routine breeding and multiplication in the SPF level animal experiment center, giving birth to male MPSII disease mice (Ids X- / Y ). Gene therapy was performed when diseased mice were 6 weeks old. We choose the adeno-associated virus dual vector system (constructed in Example 1), AAV-DJ.SaCas9: 2 × 10 11 GC and AAV-DJ.sgRNA.hIDS: 2×10 12 GC is the dosage of AAV for gene therapy of MPSII disease mouse model. We injected this amount of viral vector through the tail vein to target it to the liver of MPSII mice.
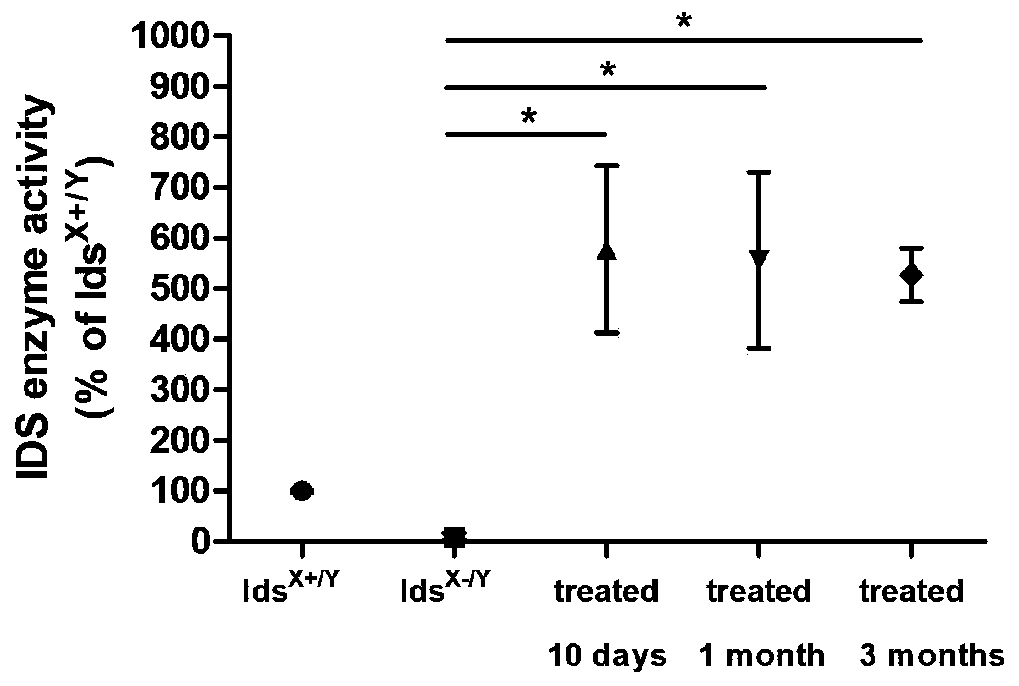
[0039] 1. Analysis of IDS enzyme activity in mouse model of MPSII disease after gene therapy
[0040]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com