Liver-targeting compound with thyroid hormone receptor stimulating agent characteristics and pharmaceutical composition of liver targeting compound
A technology of thyroid hormones and receptor agonists, which is applied in the field of targeted drugs and biomedicine, can solve problems such as limited applications, and achieve the effects of enhancing targeting, reducing distribution, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
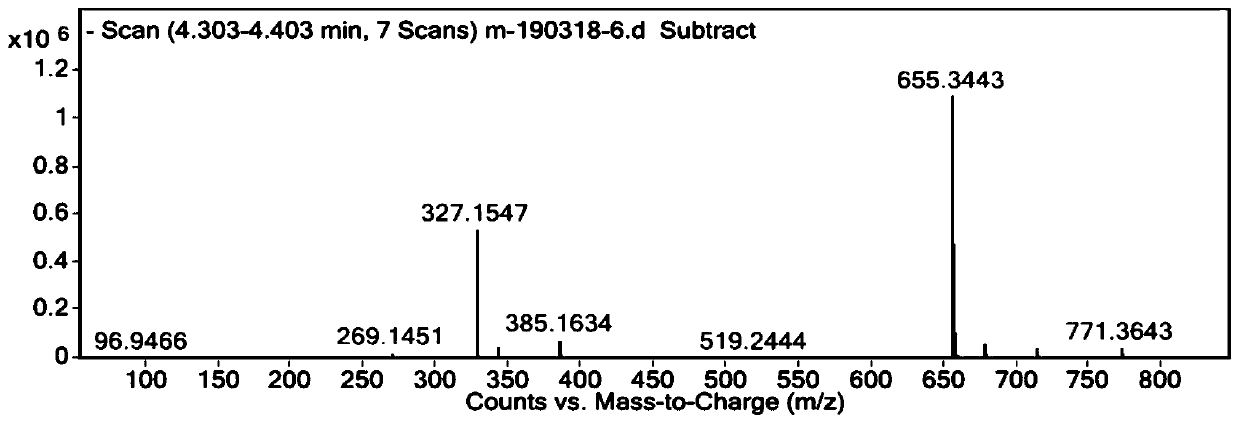
[0066] Embodiment 1: Preparation of compound GBL-0603
[0067] 1. Synthesis of Compound A
[0068] 1.1 Synthesis of Compound A-c1
[0069]
[0070] Add DMF (8mL), cbz-6-aminocaproic acid (24mg), HOBt (21.6mg), dlSANC-c12 (84mg) and DIPEA (53.5mg) successively into the reaction flask, after the addition is complete, stir the reaction at room temperature overnight, TLC Detection, the reaction is qualified, stop the reaction, perform post-processing, add water to quench, static phase separation, the aqueous phase is extracted three times with DCM, 20mL / time, the organic phase is combined, washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, concentrated , Purified by column to obtain 72.8 mg of white solid.
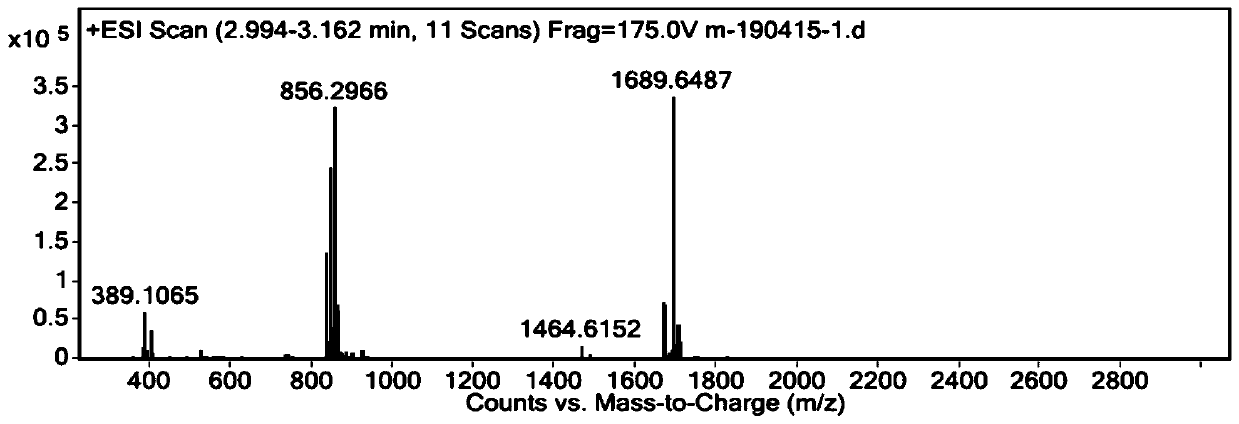
[0071] 1.2 Synthesis of compound A
[0072]
[0073] Add compound A-c1 (72.8 mg), 15 mL of methanol, and Pd / C (3.4 mg) into the reaction flask in sequence, and perform vacuum / hydrogen replacement. 2 , stirred at 40°C for 1.0 ...
Embodiment 2
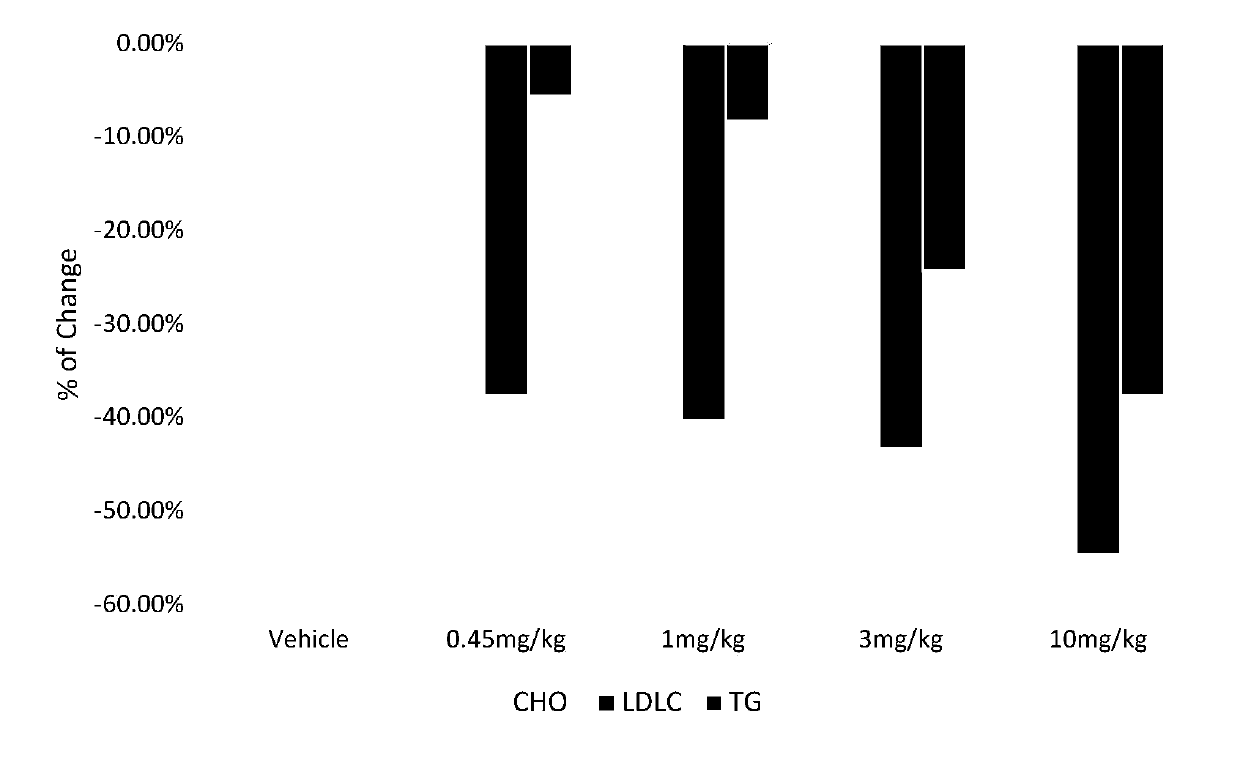
[0097] Example 2: Dosage-effect study on the effect of GBL-0603 on lipid metabolism in db mice
[0098] Experimental animals and feeding:
[0099] Thirty male 7-week-old (BKS-db) genetically obese mouse models were selected. Before the experiment, the mice had to adapt to the environment for one week, and healthy mice were selected as the experimental animals. IVC cages were raised, the feeding density was 5 / cage, and the litter was changed twice a week. Experimental animal room requirements: room temperature 22-24°C, relative humidity 40-70%, automatic lighting, 12h alternating light and dark (turn off the light at 08:00 and turn off the light at 20:00), the standard of the experimental animal room meets the national standard GB14925 of the People's Republic of China -2010.
[0100] Drug preparation method:
[0101] Accurately weigh 210 mg of GBL-0603, dissolve it in 35 mL of solvent, and prepare a 6 mg / mL mother solution. Each dosage group is sequentially diluted with th...
Embodiment 3
[0106] Embodiment 3: Add the pharmacodynamic study of accelerant sodium caprate
[0107] Animal feeding is the same as in Example 2. The grouping and dosing schedule are as follows:
[0108]
[0109] Remarks: Sodium caprate was not added to the prescription of drug A, and 20% and 75% of the weight of the main drug were added to the prescriptions of B and C respectively.
[0110] Test operation:
[0111] For G2 / G3 / G4, calculate the solvent volume according to the weight of the drug, add the solvent, vortex repeatedly, and use it after it is completely dissolved. After the preparation is complete, the administration is completed within one hour.
[0112] Before the start of the experiment, the subjects were weighed and randomly divided into groups according to body weight. Fast for 6 hours after the last administration, euthanize the mice in each group, collect blood from the heart, separate serum, detect triglyceride (TG), total cholesterol (CHO), low-density lipoprotein...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com