Synthesis method for high-purity selamectin
A technology of selamectin and a synthesis method is applied in the field of synthesis of high-purity selamectin, can solve problems such as unfavorable industrialized production, increased cost, low yield, etc. The effect of high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
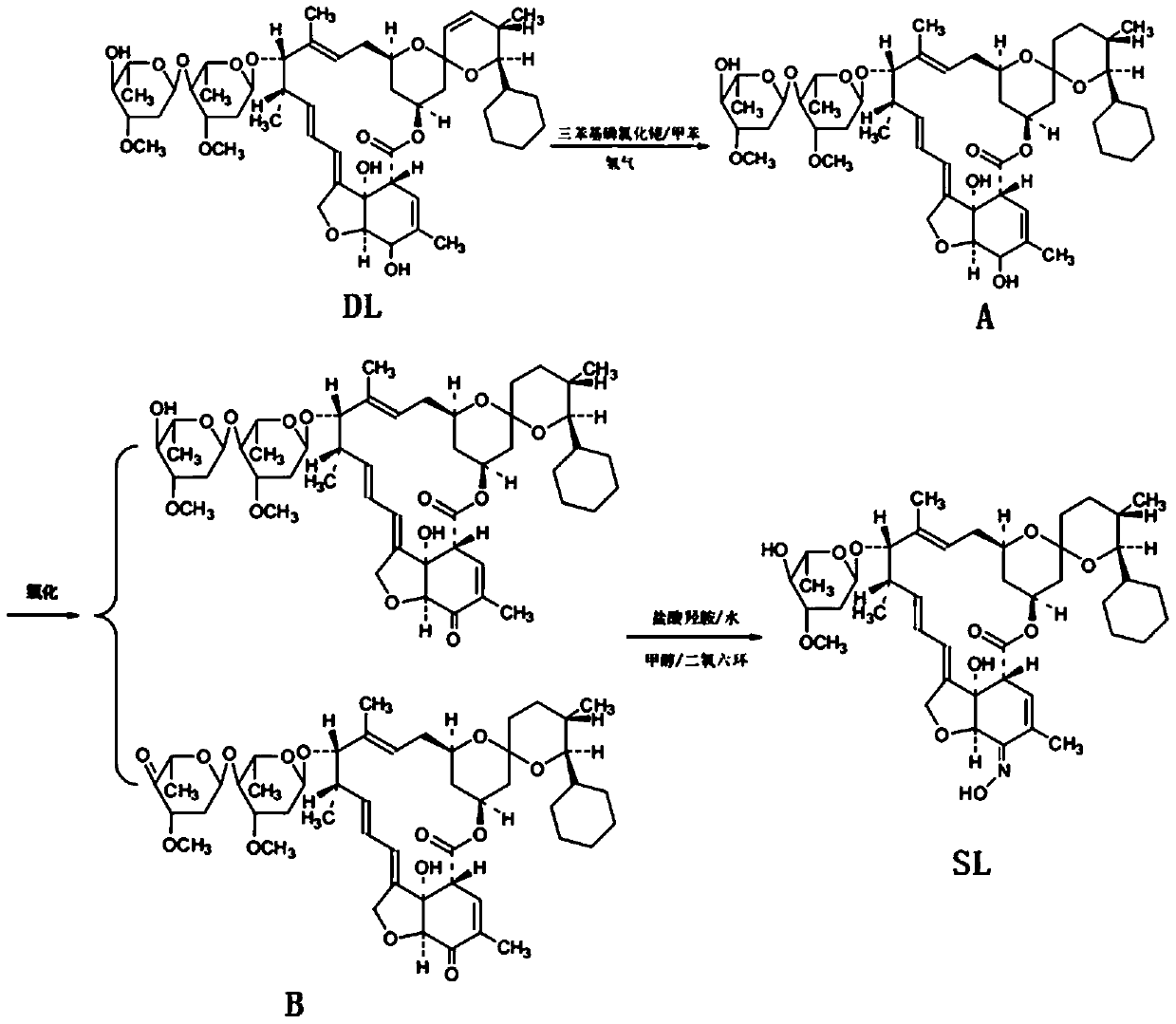
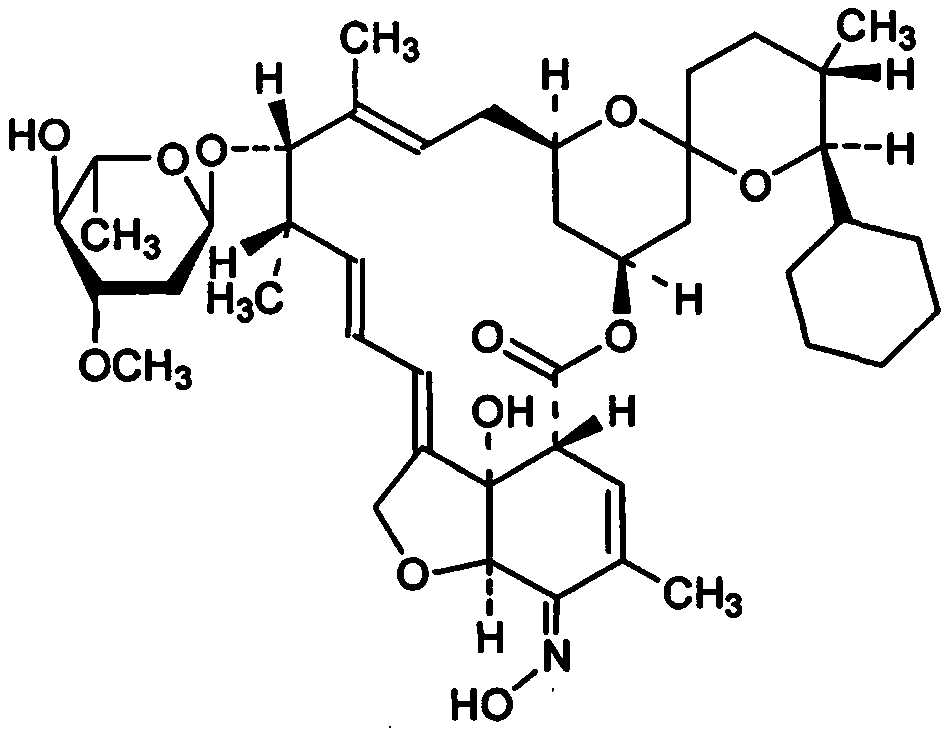
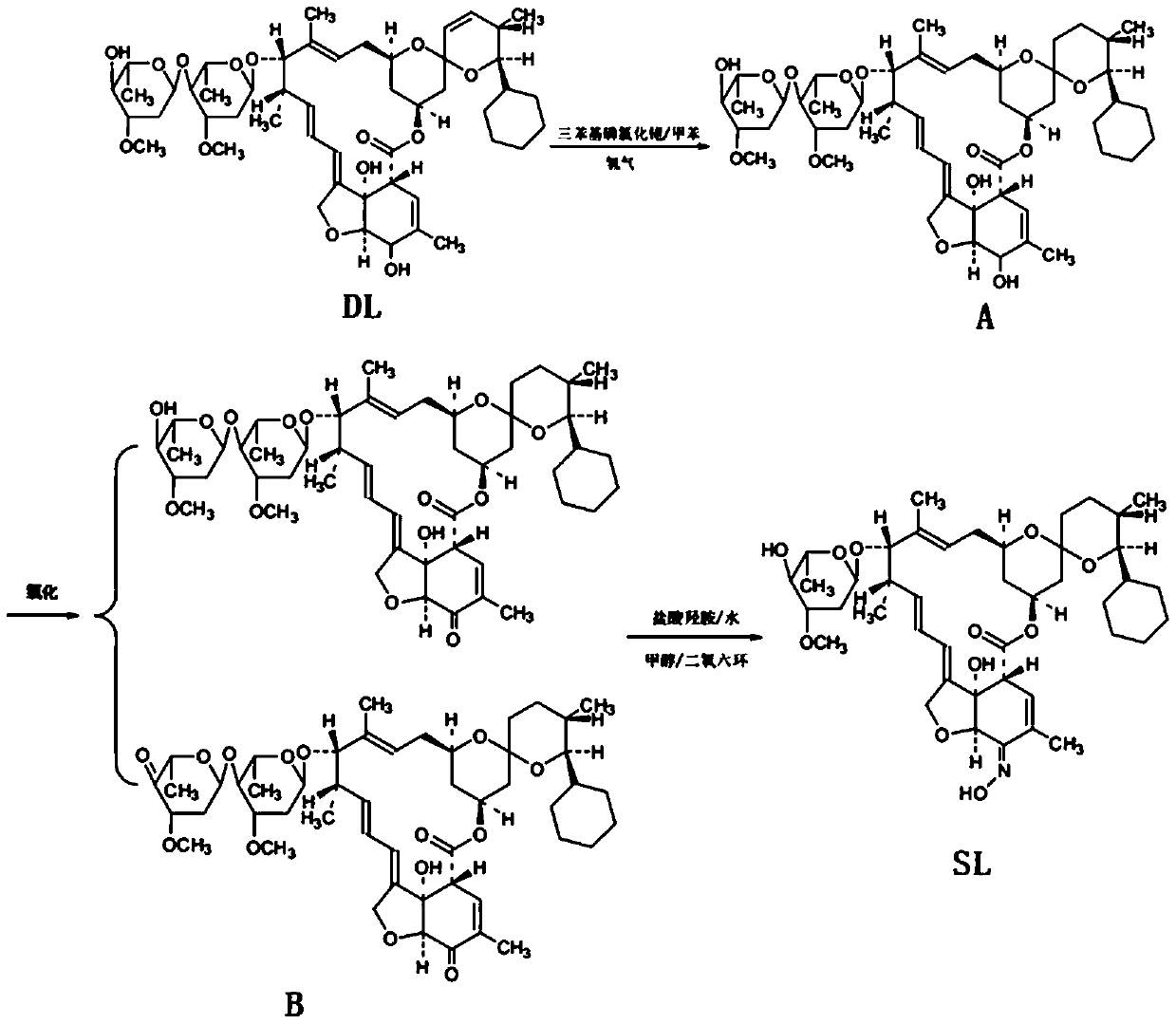
[0029] This embodiment provides a kind of synthetic method of high-purity selamectin, comprising the following steps:
[0030] (1) Preparation of Intermediate A
[0031] Add 500ml of toluene to the 1L hydrogenation autoclave that has been cleaned and tested, and add 60g of doramectin and Rh (PPh 3 ) 3 Cl 1.5g, rinse the feeding port with 100ml toluene, seal the feeding port, open the nitrogen valve, use nitrogen to raise the pressure in the kettle to 0.4MPa for three replacements, then close the nitrogen valve, open the hydrogen valve, and replace with hydrogen for three times. Finally, keep the hydrogen pressure in the kettle at about 0.4 MPa, raise the temperature and keep the temperature in the reactor at about 40°C for 4 hours. During the heat preservation process, replenish hydrogen to maintain the pressure in the kettle at 0.4 MPa. After 4 hours of heat preservation, start sampling for high-performance liquid chromatography (HPLC). Afterwards, HPLC samples are taken ev...
Embodiment 2
[0040] This embodiment provides a kind of synthetic method of high-purity selamectin, comprising the following steps:
[0041] (1) Preparation of Intermediate A
[0042] Add 500ml of toluene to the 1L hydrogenation autoclave that has been cleaned and tested, and add 60g of doramectin and Rh (PPh 3 ) 3Cl 1.5g, rinse the feeding port with 100ml toluene, seal the feeding port, open the nitrogen valve, use nitrogen to raise the pressure in the kettle to 0.4MPa for three replacements, then close the nitrogen valve, open the hydrogen valve, and replace with hydrogen for three times. Finally, keep the hydrogen pressure in the kettle at about 0.4 MPa, raise the temperature and keep the temperature in the reactor at about 40°C for 4 hours. During the heat preservation process, replenish hydrogen to maintain the pressure in the kettle at 0.4 MPa. After 4 hours of heat preservation, start sampling for high-performance liquid chromatography (HPLC). Afterwards, HPLC samples are taken eve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com