A kind of immunopotentiator and its application in vaccine preparation
A technology of immune enhancers and inactivated vaccines, applied in the field of immune enhancers and their application in vaccine preparation, and the development of therapeutic drugs, can solve the problems of reversion to ancestral strains, difficulty in forming effective protection, recombination, etc., to prevent Effects of virus invasion, high protective efficacy, and enhanced CTL response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation and identification of monoclonal antibody 5D9
[0030] 1.1 Establishment of hybridoma cell lines
[0031] The PRRSV virus SD16 (provided by the Laboratory of Immunobiology, Northwest A&F College of Veterinary Medicine) was used as an immunogen, and emulsified with Freund's complete adjuvant (Sigma) 1:1 to immunize 6-week-old female Balb / c mice (provided by Provided by Xi'an Jiaotong University), subcutaneous injection in the abdomen, the dose is (3 × 10^7 PFU / only), booster immunization once every 14 days. Seven days after the third immunization, blood was collected from the tail vein and IFA was used to detect the antibody titer against the immunogen in the serum of the mice. The mice with the best titer were immunized by tail vein injection, and the immunogen was mixed 1:1 with normal saline. , the dose is (3×10^7 PFU / only).
[0032] cell fusion
[0033] (1) Aseptically obtain the spleen cells of the immunized mice to expand the cultured mo...
Embodiment 2
[0057] Example 2: Determination of neutralizing activity of monoclonal antibody 5D9
[0058] 2.1 Neutralizing activity assay
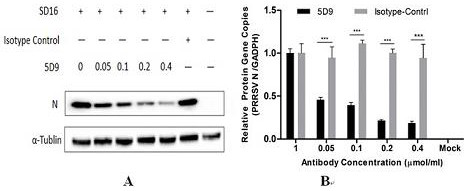
[0059] Virus neutralization experiments were performed using the monoclonal antibody 5D9 to detect its neutralization activity. PAM cells were plated into 24-well plates, and 0.01 MOI of different types of PRRSV SD16 virus were added to each virus. Each virus was added with 100 μg / ml, 200 μg / ml, 300 μg / ml, and 400 μg / ml of antibodies. After incubation at 37°C for 1 h, the cells were replaced. After 36 hours, Western blot and qPCR were performed to confirm that it had neutralizing activity. For specific results, see figure 1 .
[0060] based on figure 1 The results showed that the natural target cells of PRRSV in the host alveolar macrophages (PAMs) were cultured in vitro, and the purified monoclonal antibody 5D9 was used according to the concentration of 0.05, 0.1, 0.2, 0.4 μM / mL (micromoles per milliliter). 0.01M PRRSV-SD16 virus was incubated at...
Embodiment 3
[0067] 3.1 Preparation of antigen and complexation with antibody (take PRRSV virus as an example below)
[0068] Use PRRSV-SD16 strain to infect Marc145 cells, collect virus culture supernatant 72 hours after virus infection, centrifuge a sufficient amount of virus culture supernatant at 12000g for 1 hour to remove cell debris, and use 100kD molecular weight through Labscale TFF filter. The filter (EMD Millipore) was concentrated 50-fold using a 100kD molecular weight filter, and the PRRSV-SD16 virions were purified by liquid chromatography.
[0069] The virus was treated with β-propiolactone at a concentration of 1 / 1000 and placed at 4 degrees overnight to inactivate the virus. After being placed in a water bath at 37 degrees for 1 hour to degrade β-propiolactone, the virus was mixed with monoclonal antibody 5D9 (calculated by mass). ) were mixed at a ratio of 1:5 and placed at 37°C for 2 hours to form a complex.
[0070] Preparation of vaccines and immunization of mice
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com