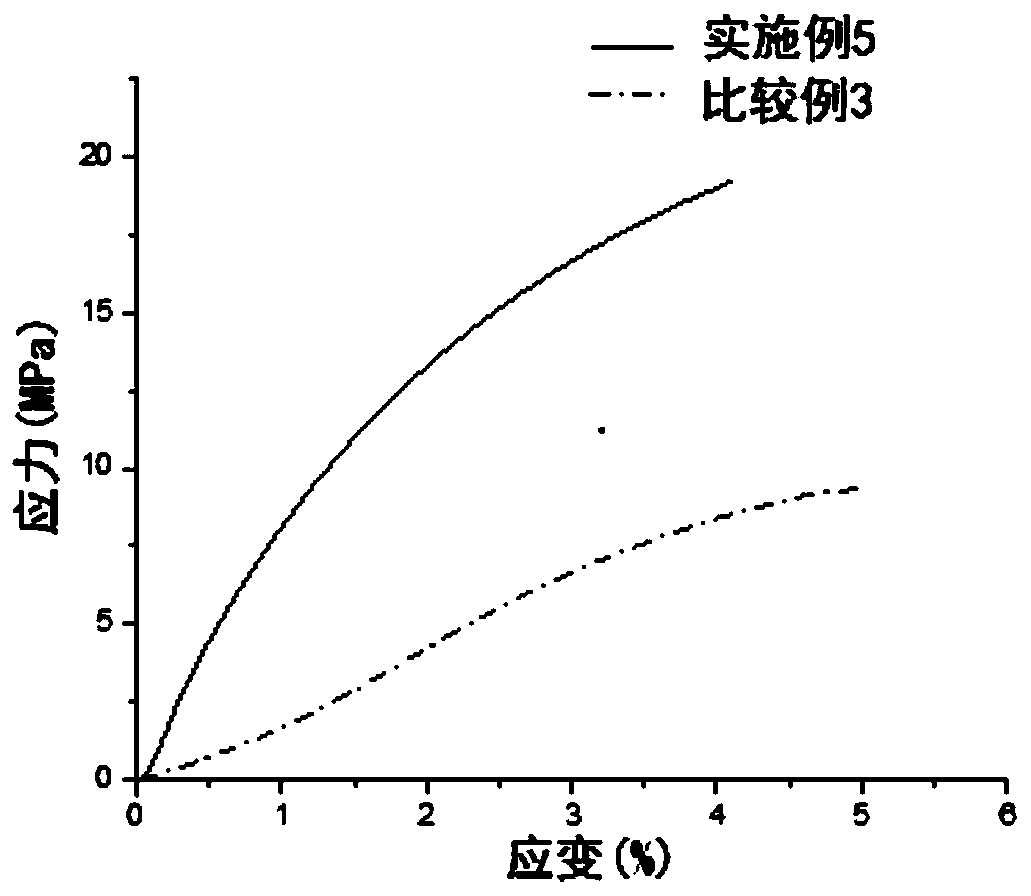
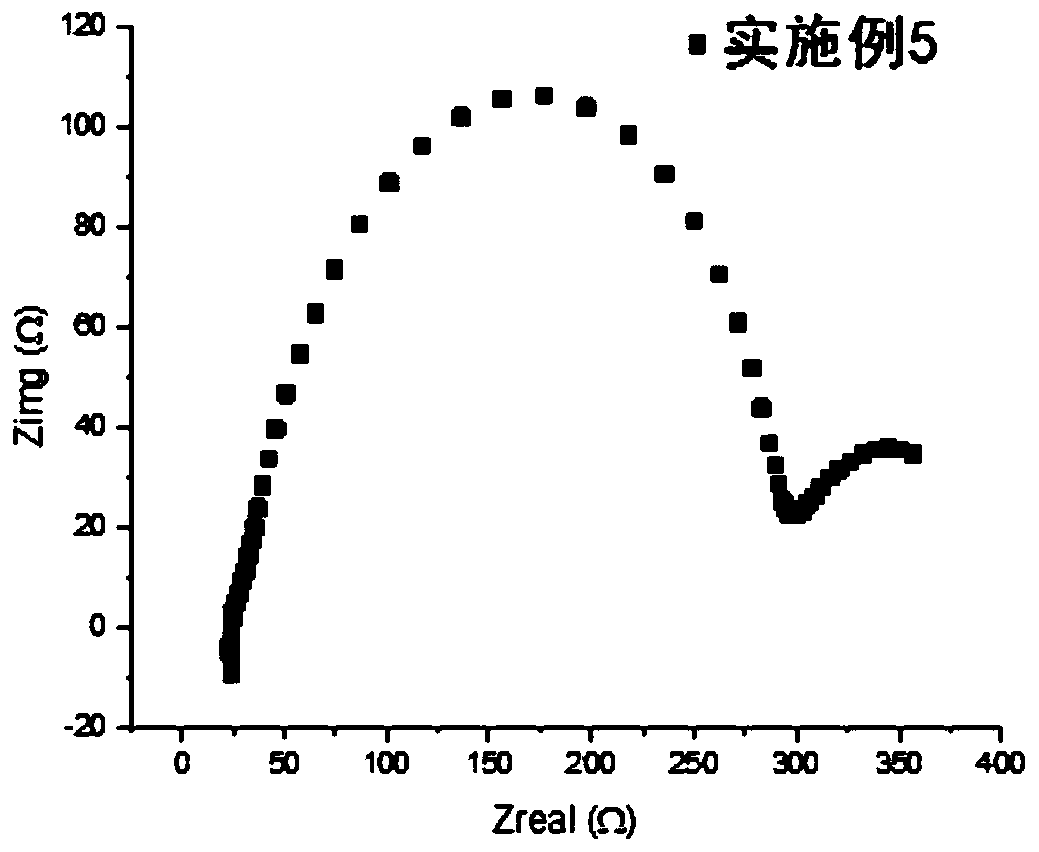
Polymer solid electrolyte, method of making the same, and electrochemical cell
A solid electrolyte, polymer technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, solid electrolytes, etc., can solve problems such as combustion or even explosion, LIBs fire, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] This example shows the synthesis of a PEG-urethane-diepoxy polymer having the general structure:
[0077]
[0078] In this example, 40 mmol of 4,7,10-trioxa-1,13-tridecanediamine (TTDDA; commonly known as polyethylene glycol diamine (PEGDAm)), 200 mmol of pyridine and 100 ml of CHCl3 Add to 250 mL round bottom flask. The mixture was stirred at -10 °C for 10 min, then 80 ml of 85 mmol triphosgene in CHCl was added within 10 min at -20 °C 3 The solution was added to the solution. These are generally all commercially available; in particular, PEGDAm is available in a molecular weight range corresponding to the number of repeating units of polyethylene glycol within the compound. see also Figure 5 .
[0079] The mixture was stirred at 0 °C for 5 h, then a pale yellow solution was obtained. 100ml of 0.1mol / l hydrochloric acid was poured into the reaction mixture, and the product was collected and washed with 200ml CHCl 3 Wash twice. Using MgSO 4 After drying, the ...
Embodiment 2
[0087] This example shows the synthesis of PEG-urea-diepoxy polymers having the general structure:
[0088]
[0089] In this example, 40 mmol of 4,7,10-trioxa-1,13-tridecanediamine (TTDDA; commonly known as polyethylene glycol diamine (PEGDAm)), 200 mmol of pyridine and 100 ml of CHCl 3 Add to 250 mL round bottom flask. The mixture was stirred at -10 °C for 10 min, then 80 ml of 85 mmol triphosgene in CHCl was added within 10 min at -20 °C 3 The solution was added to the solution. The mixture was then stirred at 0 °C for 5 h. A pale yellow solution was obtained.
[0090] 100 ml of 0.1 mol / L hydrochloric acid was added to the reaction mixture to obtain a two-phase separated liquid mixture. The product was collected and washed with 200ml CHCl 3 Wash twice. By adding MgSO 4 Remove moisture from the product. After evaporation of the solvent, a light brown crude diisocyanate is obtained. The product (PEG-diisocyanate or PEGDI) was purified by silica gel column.
[0091...
Embodiment 3
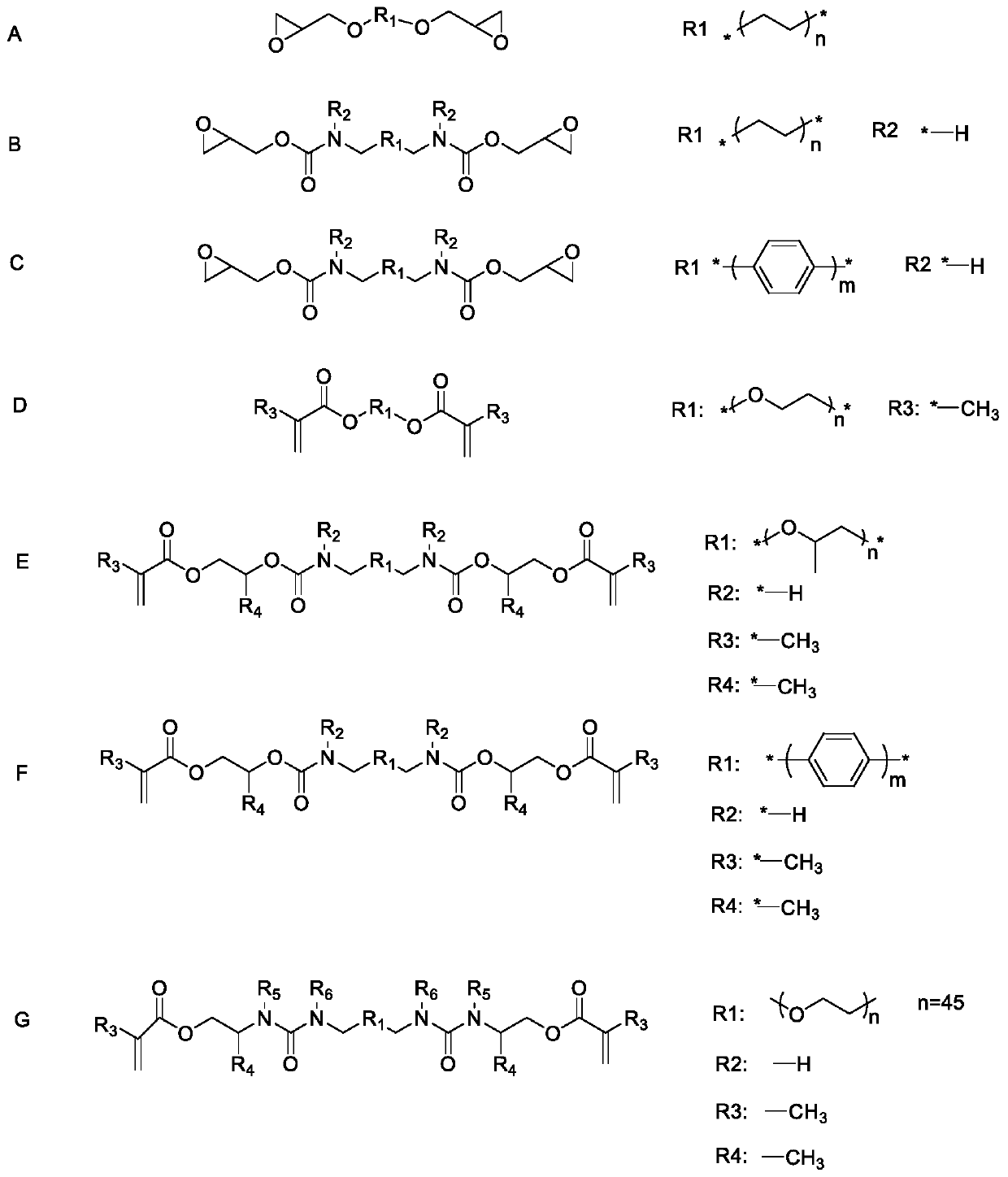
[0095] This example shows the synthesis of decyl-polyurethane-diepoxy, polymer B (see image 3 ):
[0096]
[0097] In this structure, n is 5. In this example, 60mmol 1,10-diaminodecane, 120mmol pyridine and 50ml CHCl 3 Add to 100ml round bottom flask. The mixture was stirred at -20°C for 30 min, then 80 ml of 85 mmol triphosgene in CHCl 3 The solution was added to the solution at -20°C within 10 min. The mixture was then stirred at 0 °C for 12 h. A pale yellow solution was obtained.
[0098] 100 ml of 0.1 mol / l hydrochloric acid was added to the reaction mixture to obtain a two-phase separated liquid mixture. The product was collected and washed with 200ml CHCl 3 Wash twice. By adding MgSO 4 Remove moisture from the product. After evaporation of the solvent, a light brown crude diisocyanate is obtained. The product (decyl diisocyanate or DDI) was purified by silica gel column.
[0099] 10mmol DDI and 25mmol glycidol were reacted at 0°C for 24h under the catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com