Inorganic-organic nano composite solid electrolyte membrane and preparation method and application thereof
A solid electrolyte and nanocomposite technology, which is used in solid electrolytes, electrolyte battery manufacturing, non-aqueous electrolytes, etc., to achieve high Coulomb efficiency and cycle life, reduce interface resistance, and inhibit lithium dendrites.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
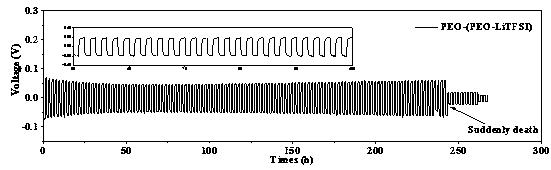
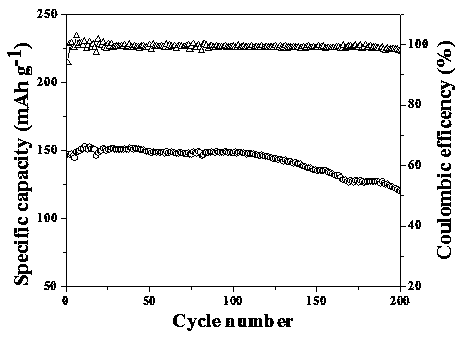
[0035] (1) Preparation of Li by solid-state reaction method 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 (LATP) Inorganic ceramic solid electrolyte powder. Lithium carbonate (Li 2 CO 3 ), aluminum oxide (Al 2 o 3 ), titanium dioxide (TiO 2 ), ammonium monohydrogen phosphate ((NH 4 ) 2 HPO 4 ) and grind to mix. In order to compensate for the loss of lithium salt in the heat treatment process, lithium carbonate is in excess of 10%. After sintering at 900 °C for 2 h, ball milling at 400 rpm for 6 h with acetone as solvent, then calcining at 900 °C for 2 h and ball milling for 6 h to obtain LATP powder. (2) Polyethylene oxide (PEO, molecular weight 400,000) and lithium salt (LiTFSI) were vacuum-dried at 60 °C and 100 °C overnight before use, and then 3 g of PEO and 1 g of LiTFSI were dissolved in 40 mL of acetonitrile (AN) , mechanically stirred at 60°C for 12 h, and then coated on the washed polymer non-woven PET, dried at 60°C and rolled to obtain an interface layer thickness of a...
Embodiment 2
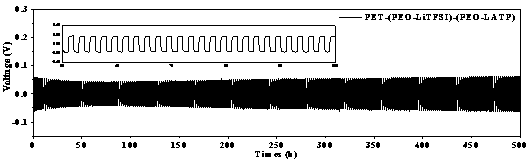
[0037] (1) Preparation of Li by solid-state reaction method 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 (LATP) Inorganic ceramic solid electrolyte powder. Lithium carbonate (Li 2 CO 3 ), aluminum oxide (Al 2 o 3 ), titanium dioxide (TiO 2 ), ammonium monohydrogen phosphate ((NH 4 ) 2 HPO 4 ) and grind to mix. In order to compensate for the loss of lithium salt in the heat treatment process, lithium carbonate is in excess of 10%. After sintering at 900 °C for 2 h, ball milling at 400 rpm for 12 h with acetone as solvent, then calcining at 900 °C for 2 h and ball milling for 12 h to obtain LATP powder. (2) Polyethylene oxide (PEO, molecular weight 400,000) and lithium salt (LiTFSI) were vacuum-dried at 60 °C and 100 °C overnight before use, and then 3 g of PEO and 1 g of LiTFSI were dissolved in 40 mL of acetonitrile (AN) , mechanically stirred at 60 °C for 12 h, and then coated on the washed polymer non-woven PET, dried at 60 °C and then rolled to obtain an interface layer thick...
Embodiment 3
[0039] (1) Preparation of Li by solid-state reaction method 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 (LATP) Inorganic ceramic solid electrolyte powder. Lithium carbonate (Li 2 CO 3 ), aluminum oxide (Al 2 o 3 ), titanium dioxide (TiO 2 ), ammonium monohydrogen phosphate ((NH 4 ) 2 HPO 4 ) and grind to mix. In order to compensate for the loss of lithium salt in the heat treatment process, lithium carbonate is in excess of 10%. After sintering at 900 °C for 2 h, ball milling at 400 rpm for 6 h with acetone as solvent, then calcining at 900 °C for 2 h and ball milling for 6 h to obtain LATP powder. (2) Polyethylene oxide (PEO, molecular weight 400,000) and lithium salt (LiTFSI) were vacuum-dried at 60 °C and 100 °C overnight before use, and then 3 g of PEO and 1 g of LiTFSI were dissolved in 40 mL of acetonitrile (AN) , mechanically stirred at 60 °C for 12 h, and then coated on the washed polymer non-woven PET, dried at 60 °C and then rolled to obtain an interface layer thickne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com