Ziprasidone hydrochloride pharmaceutical composition and preparation method thereof
A technology for ziprasidone hydrochloride and composition, which is applied in the field of ziprasidone hydrochloride pharmaceutical composition and preparation thereof, can solve the problems of decreased drug efficacy, poor medication compliance, poor solubility and the like, and achieves improved dissolution performance and drug stability. Good performance and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
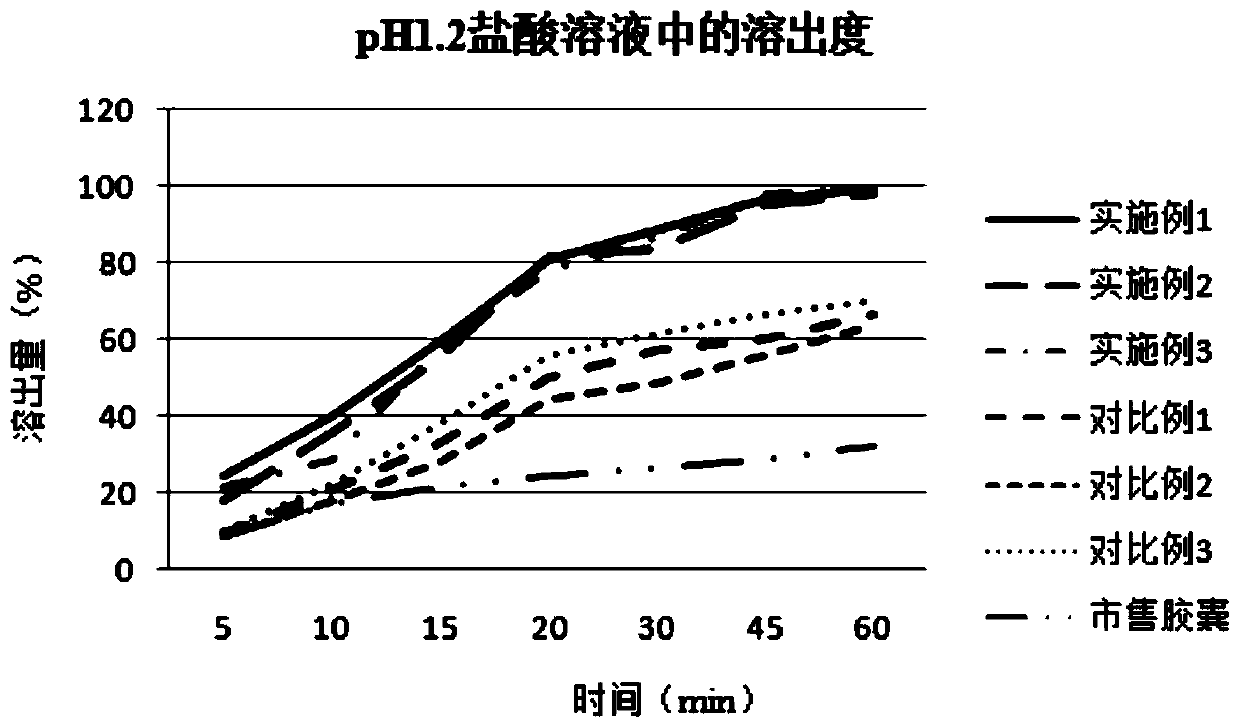
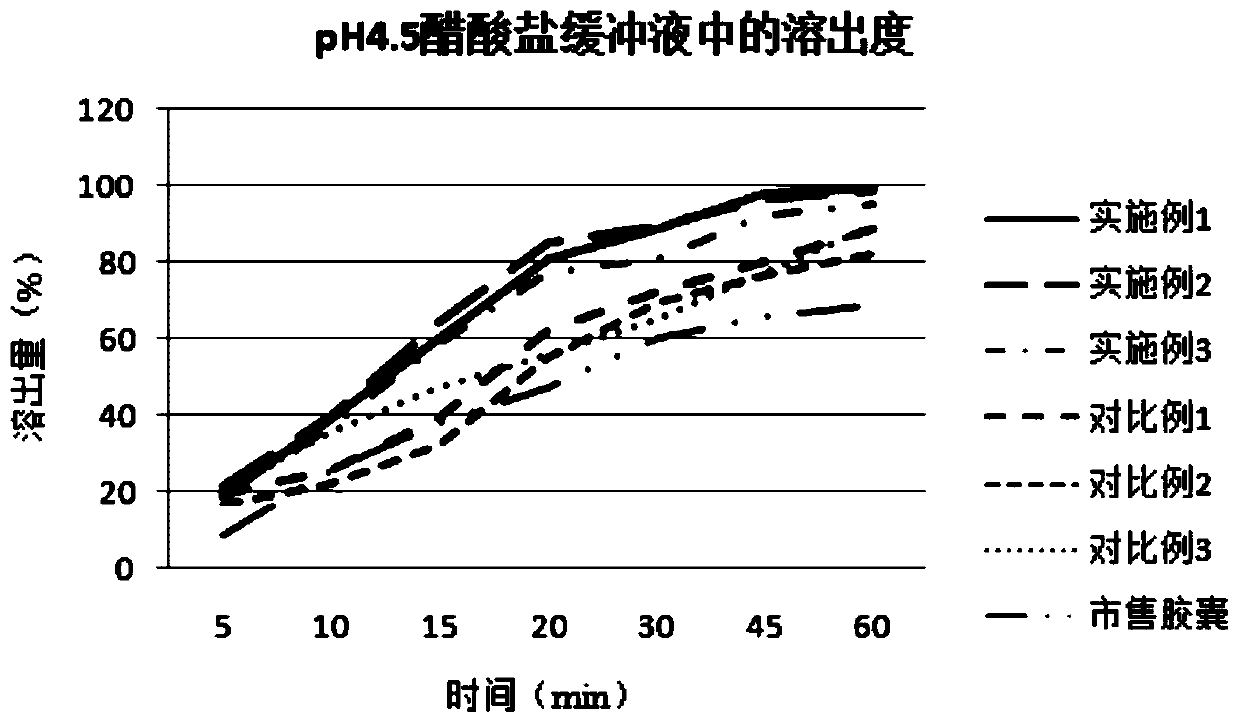
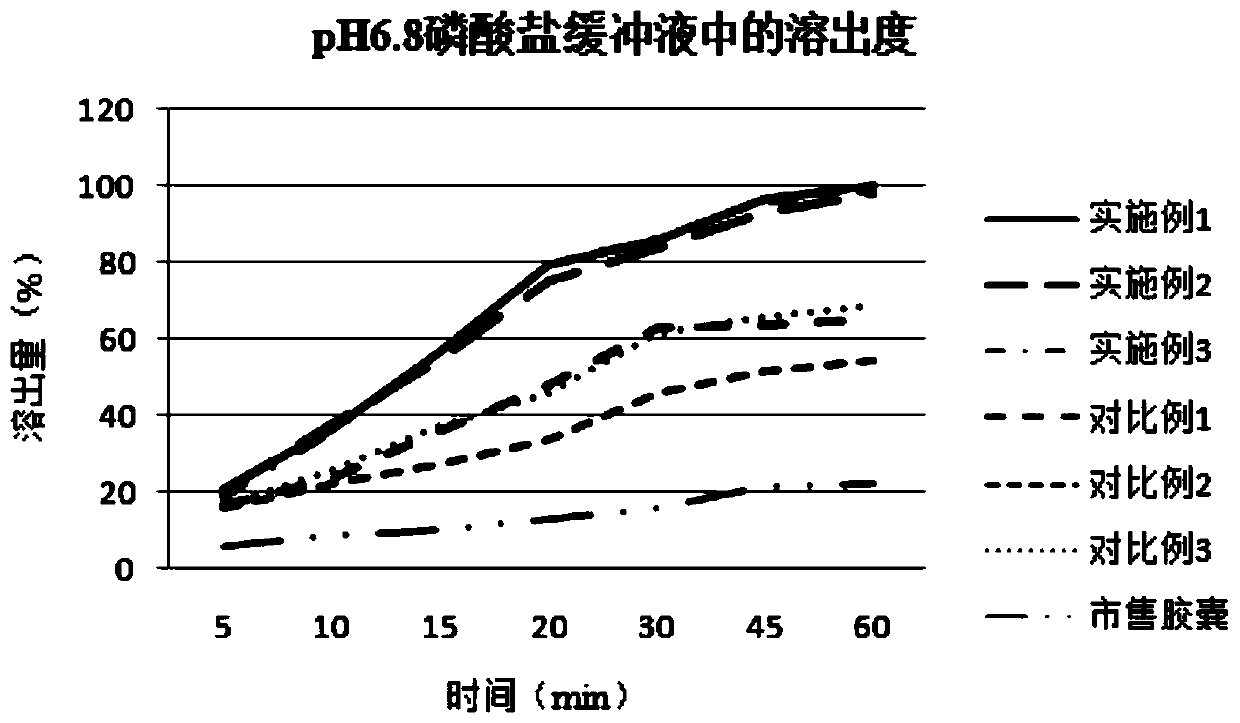
[0038] Embodiment 1: a kind of ziprasidone hydrochloride pharmaceutical composition, its prescription composition and preparation process are as follows:
[0039] components Dosage (g) Ziprasidone hydrochloride 10 glutamic acid 0.1 Sodium acetate 1 lactose 5 hypromellose 5
[0040] Preparation method: Add ziprasidone hydrochloride into water according to the formula quantity, heat to 45°C, and stir at 150rpm to make the drug into a suspended state to make drug suspension A, and add the remaining components into an appropriate amount of water until dissolved to prepare Solution B. While stirring, solution B was slowly added to solution A, and the stirring was continued for 30 min to dissolve ziprasidone hydrochloride to obtain reddish mixed solution C.
[0041] The mixed solution C was evaporated to dryness under vacuum at 45° C. to obtain material D. The material D was placed in a vacuum drying oven at 40° C. for 24 hours in vacu...
Embodiment 2
[0043] Embodiment 2: A kind of ziprasidone hydrochloride pharmaceutical composition
[0044] components Dosage (g) Ziprasidone hydrochloride 10 glutamic acid 0.5 Sodium acetate 5 lactose 8 hypromellose 8
[0045] The preparation method refers to Example 1.
Embodiment 3
[0046] Embodiment 3: A kind of ziprasidone hydrochloride pharmaceutical composition
[0047] components Dosage (g) Ziprasidone hydrochloride 10 glutamic acid 1 Sodium acetate 10 lactose 10 hypromellose 10
[0048] The preparation method refers to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com