Application of miRNA in treating cardiac hypertrophy
A kind of myocardial hypertrophy, CHO-PGEA- technology, applied in the field of medicine and biology, can solve problems such as poor clinical effect of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Supplementation of hsa-miR-15a-5p / hsa-miR-16-5p (delivered by CHO-PEGA cation carrier) in vitro inhibits cardiac hypertrophy
[0037] The therapeutic effect of hsa-miR-15a-5p / hsa-miR-16-5p supplementation was tested.
[0038] Culture embryonic rat cardiomyocyte cell line (H9C2 cell line) in DMEM supplemented with 10% heat-inactivated fetal calf serum and antibiotics, culture embryonic rat cardiomyocyte cell line (H9C2 cell line) in a 24-well plate, and then add 20 μL CHO-PGEA-miRNA complex.
[0039] The preparation of the CHO-PGEA-miRNA complex can refer to the previous work of the applicant (such as CN201510377896.X, CN201910327563.4): mix the ingredients in the following table 3, shake for 5 seconds and then let stand for 30 minutes:
[0040] Table 3. Components of CHO-PGEA-miRNA complexes
[0041]
[0042] Cells were divided into negative control group and experimental group.
[0043] (1) Negative control group: 20 μL LCHO-PEGA-mirNC (no functional m...
Embodiment 2
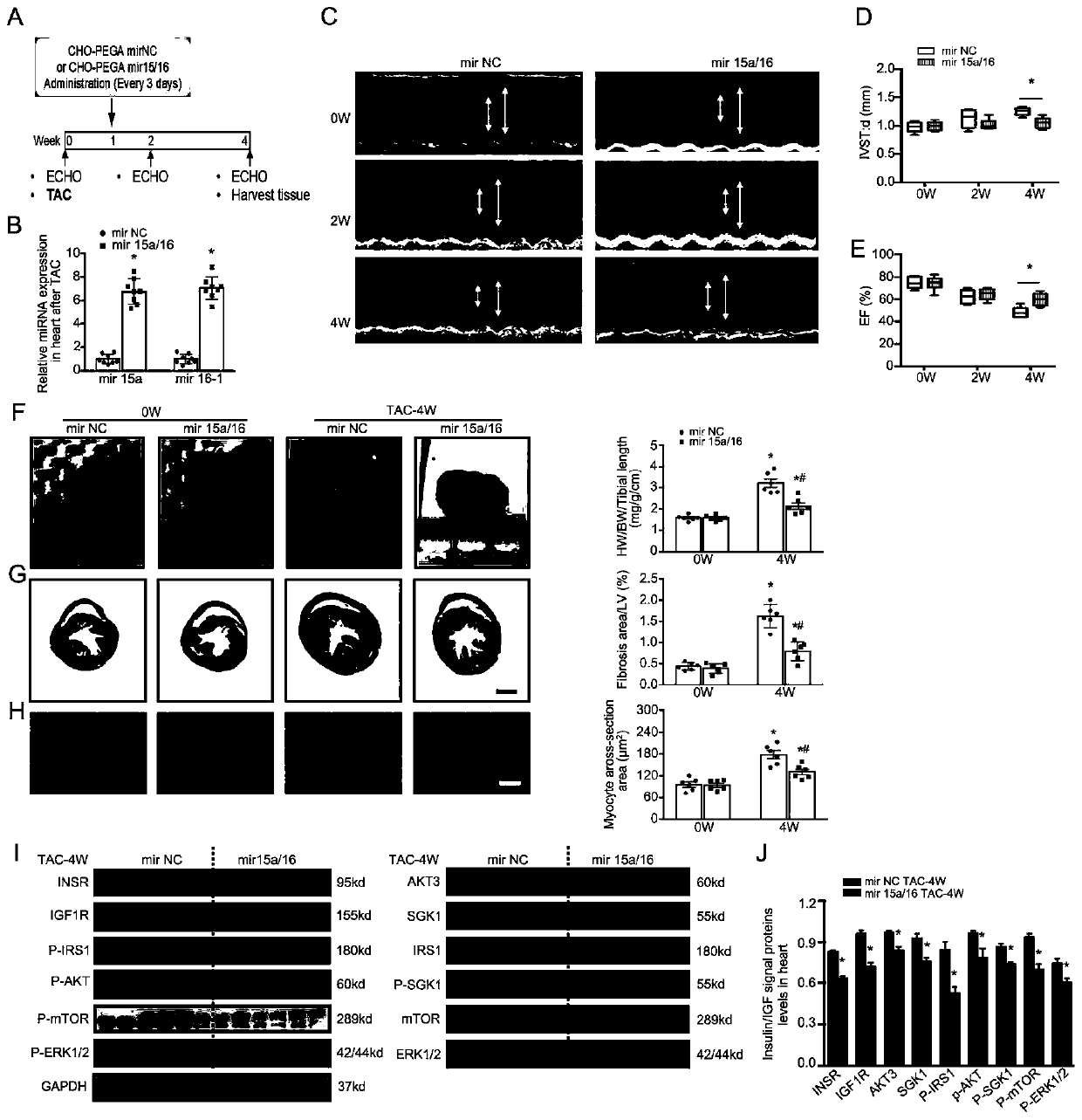
[0058] Example 2. In vivo supplementation of hsa-miR-15a-5p / hsa-miR-16-5p (delivered by CHO-PEGA cation carrier) can completely inhibit TAC-induced cardiac hypertrophy and improve myocardial fatty acid oxidation
[0059] 1. Modeling steps of mouse TAC model:
[0060] (1) The mice were fixed under induction anesthesia. Maintain anesthesia with 1.5% isoflurane mixed gas through tracheal intubation;
[0061] (2) Disinfect the surgical area and prepare the skin for thoracotomy. Exposure of the thoracic aorta after thoracotomy;
[0062] (3) At the aortic arch, a 27G thin needle was placed parallel to the aortic arch and ligated together;
[0063] (4) After the ligation, draw out the fine needle to observe the condition of the mouse, and suture layer by layer after the condition is stable.
[0064] 2. Experimental grouping and scheme ( figure 2 A)
[0065] Select 15 wild mice and divide them into 3 groups, including:
[0066] (1) 5 mice in the blank control group: inject nor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com