All-vanadium redox flow battery electrolyte formula and process for inhibiting precipitation of easily precipitated element impurities in electrolyte
An all-vanadium redox flow battery and elemental impurity technology, applied in electrolytes, acidic electrolytes, aqueous electrolytes, etc., can solve the problems of reduced discharge capacity, aggravated self-discharge of the battery system, and unbalanced charge of the negative electrolyte, achieving raw materials and Effect of production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
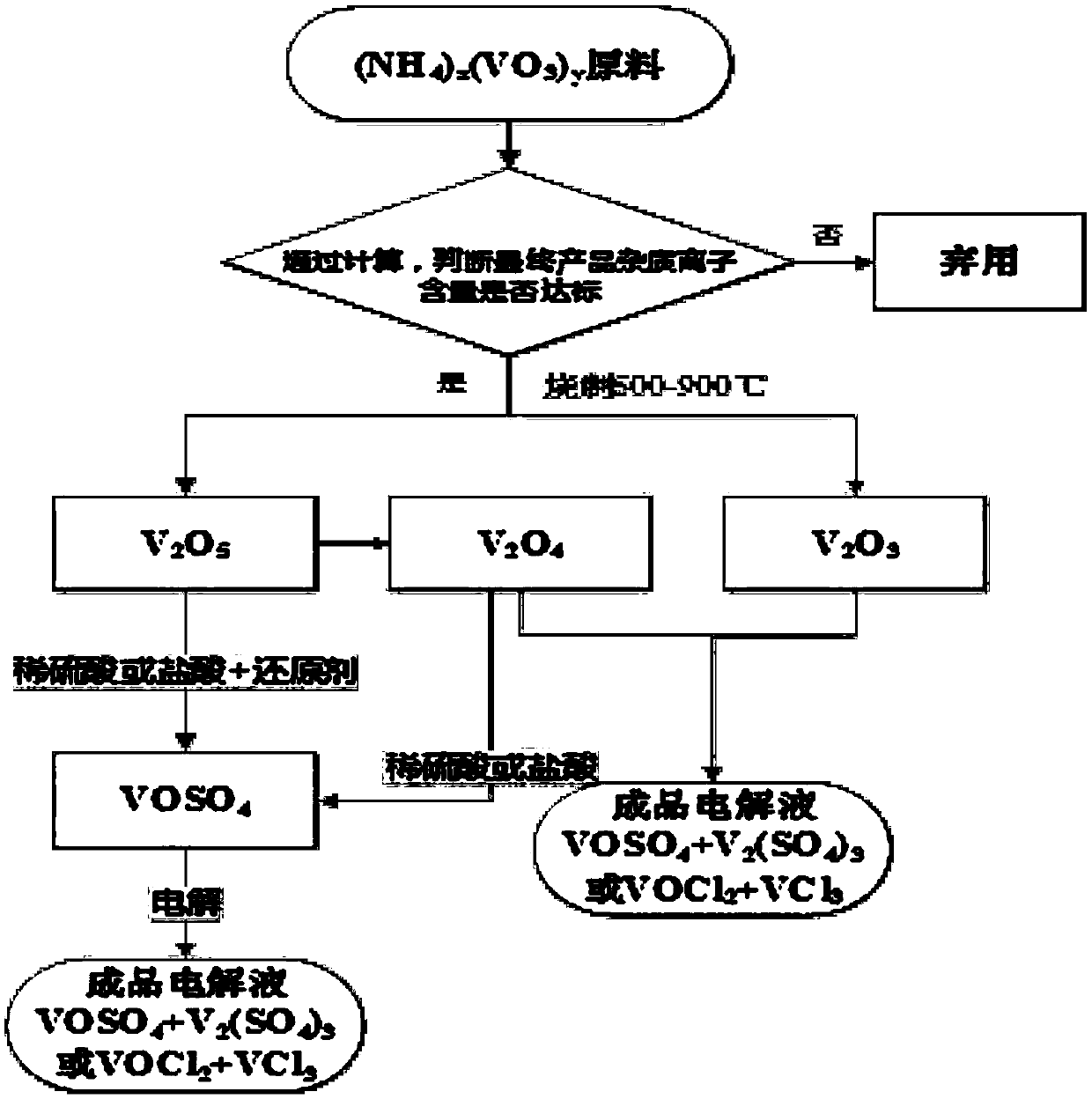
Image
Examples
Embodiment 1
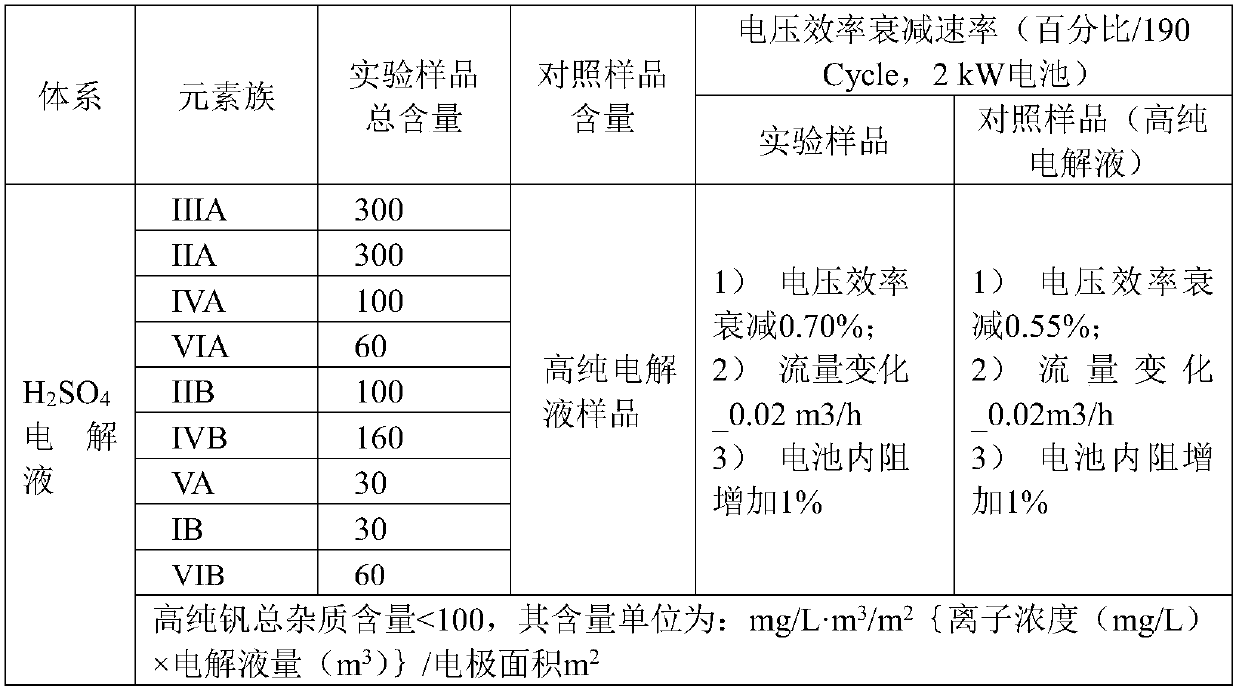
[0060] The vanadium electrolyte was prepared and operated according to the content of the experimental sample and the control sample in the table below, and the results obtained were as shown in the table below:
[0061]
[0062] The above data and operation results show that after properly amplifying the concentrations of the above 9 types of easy-to-deposit elements, the H 2 SO 4 The system electrolyte, 2kW battery, has experienced more than 190 charge and discharge cycles. The experimental electrolyte system has no significant change in system efficiency compared with the control high-purity vanadium sample battery, indicating that the easy-to-deposit elements are properly released, and the long-term charge and discharge operation of the system. Discharge capacity, Efficiency etc. have no effect.
Embodiment 2
[0064] The vanadium electrolyte was prepared and operated according to the content of the experimental sample and the control sample in the table below, and the results obtained were as shown in the table below:
[0065]
[0066]
[0067] The above data and operation results show that the H 2 SO 4 System electrolyte, 2kW battery, after more than 190 charge and discharge cycles, the experimental electrolyte with the concentration of the above 9 types of easy-to-deposit elements appropriately amplified, the efficiency of the sample electrolyte battery with a higher concentration of deposition elements than the control remains good, and the flow rate is not obvious Changes, the internal resistance of the control electrolyte system has increased significantly, indicating that the elements that are easy to deposit are excessively released, which has a serious impact on the discharge capacity and efficiency of the long-term charging and discharging operation of the system.
Embodiment 3
[0069] The vanadium electrolyte was prepared and operated according to the content of the experimental sample and the control sample in the table below, and the results obtained were as shown in the table below:
[0070]
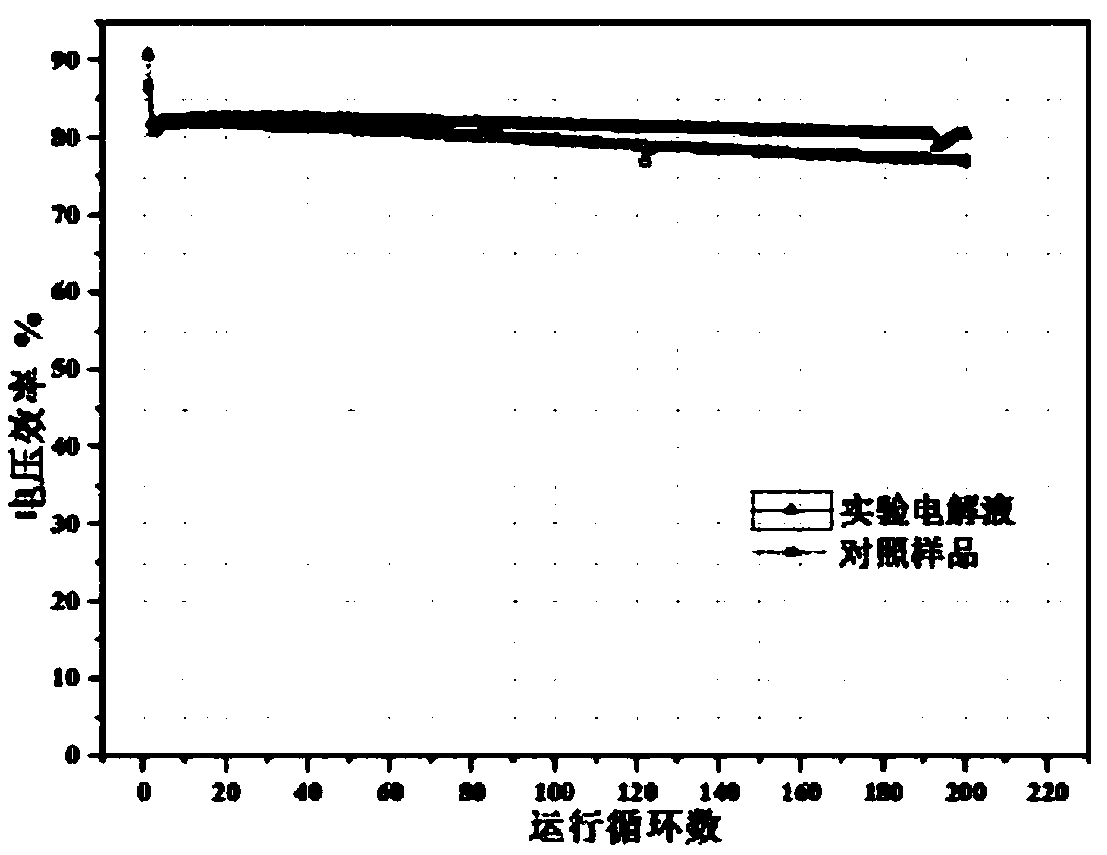
[0071] The above data and operation results show that after properly amplifying the concentrations of the above group 3 easy-to-deposit elements, the H 2 SO 4 System electrolyte, 10kW battery, after more than 190 charge and discharge cycles, the experimental electrolyte system is 6 points higher than the system efficiency of the control sample battery ( figure 2 ), indicating that the excessive release of easy-to-deposit elements has a serious impact on the discharge capacity and efficiency of the long-term charging and discharging operation of the system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com