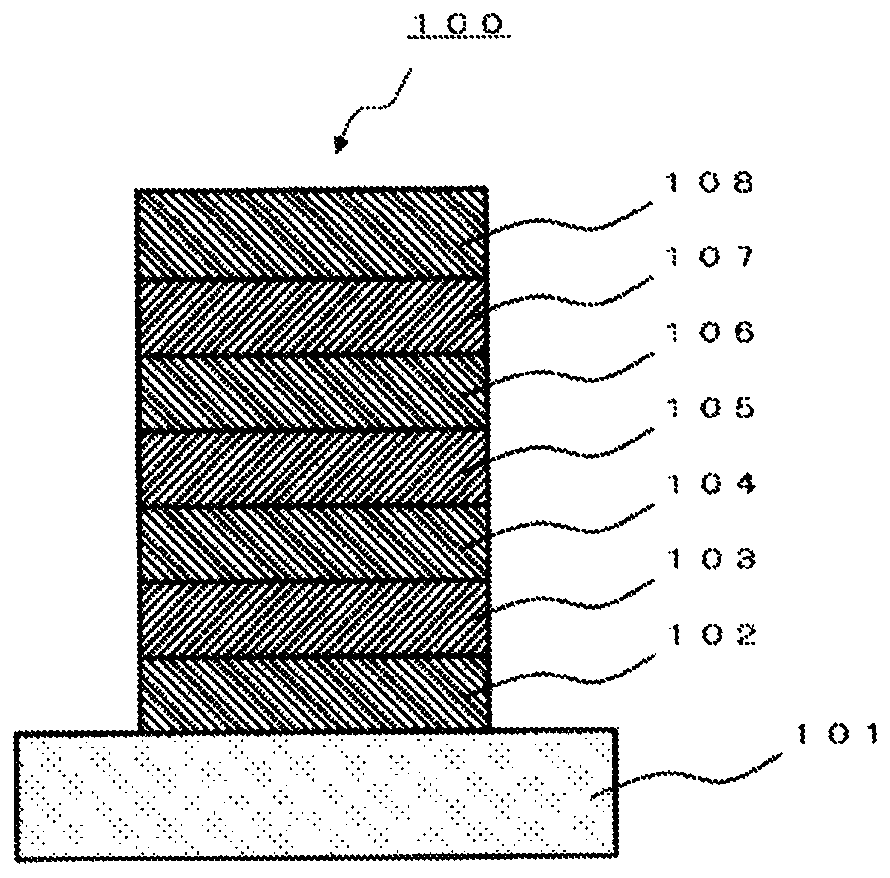
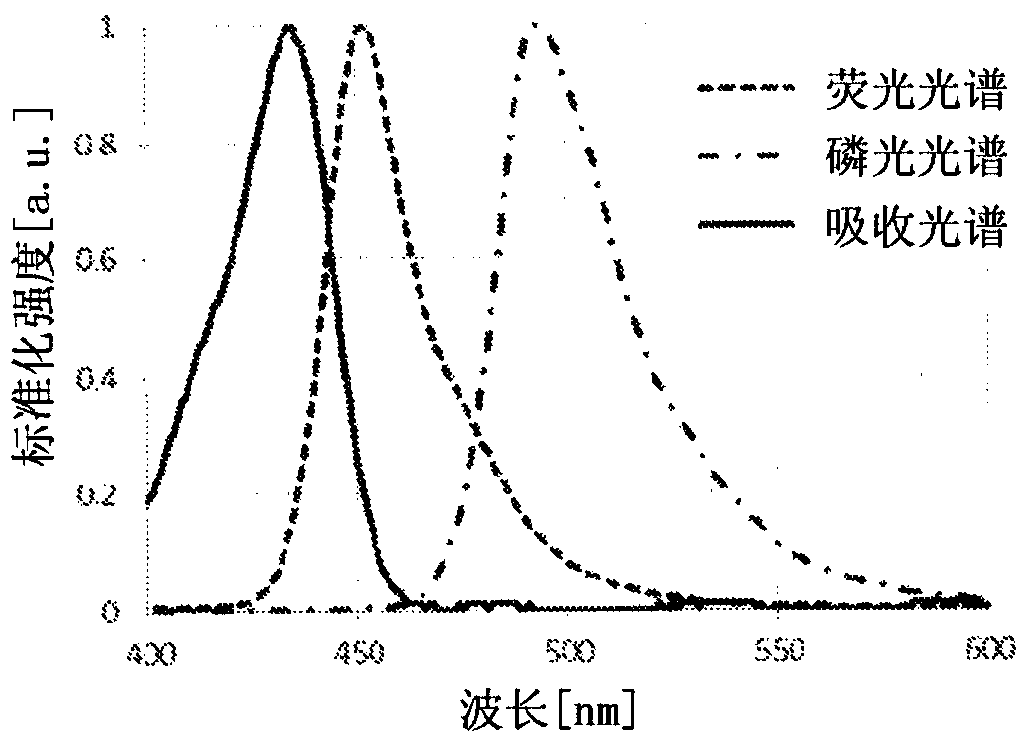
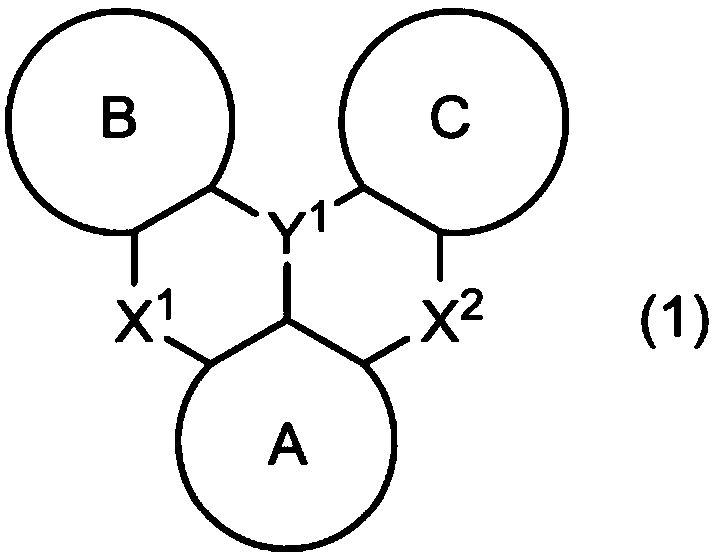
Polycyclic aromatic dimeric compound
A compound and dimer technology, applied in the field of display devices and lighting devices, can solve the problems of insufficient life, unsuitable main material, insufficient redox stability of aromatic epoxies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0664] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to these. First, a synthesis example of a polycyclic aromatic dimer compound will be described below.
Synthetic example (1
[0666] Compound (1-201): 1,3-bis(5,9-diphenyl-5,9-dihydro-5,9-diaza-13bborinaphtho[3,2,1-de] Synthesis of anthracene-7-yl)thio)benzene
[0667] [CH215]
[0668]
[0669] [paragraph 1]
[0670] Under nitrogen atmosphere, 1,3-diiodobenzene (4.48g, 14mmol), 3,5-dibromobenzenethiol (5.50g, 31mmol), potassium carbonate (4.27g, 31mmol) were dissolved in 150mL of N, In N-dimethylformamide (N,N-Dimethylformamide, DMF), copper iodide (0.286g, 1.5mmol) was added thereto, and after stirring at 100°C for 4 hours, the reaction solution was cooled, and The solvent was distilled off to obtain a crude product. The crude product obtained using silica gel was filtered (eluent: hexane), and the residue was washed with hexane using an ultrasonic pulverizer to obtain the target 1 as a white solid. 3-Bis((3,5-dichlorophenyl)thio)benzene (4.73 g, 81% yield).
[0671] [chem 216]
[0672]
[0673] The structures of the obtained compounds were confirmed by nuclear magnetic resonance (Nuclea...
Synthetic example (2
[0689] Compound (1-5400): 1,3-bis(5,9-diphenyl-5,9-dihydro-5,9-diaza-13bborinaphtho[3,2,1-de] Synthesis of anthracen-7-yl)oxy)benzene
[0690] [chem 219]
[0691]
[0692] [paragraph 1]
[0693] Under nitrogen atmosphere, dissolve resorcinol (11.6g, 105mmol), 1-bromo-3,5-dichlorobenzene (55.0g, 221mmol), potassium carbonate (4.27g, 31mmol) in 180mL of N-methyl Base-2-pyrrolidone (N-Methyl-2-Pyrrolidone, NMP), copper iodide (4.01g, 21.1mmol), tris (2,4-pentanedionyl) iron (III) (7.44g , 21.1mmol), triphenylphosphine (22.1g, 84.3mmol), and after stirring at 180° C. for 4 hours, the reaction solution was cooled and filtered with celite. The filtrate was washed 3 times with water, and dried with anhydrous sodium sulfate. The constituent organisms obtained by concentrating the obtained solution were purified by silica gel chromatography (toluene), and then concentrated to obtain 1,3-bis(3 , 5-dichlorophenoxy)benzene (15.5 g, 37% yield).
[0694] [Chemical 220]
[0695] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Light wavelength | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com