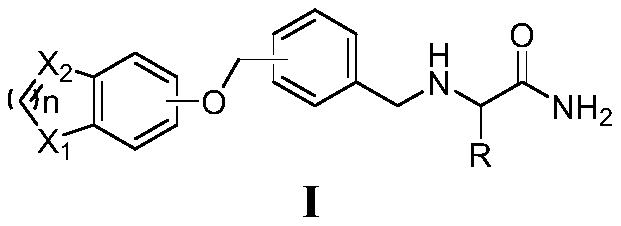
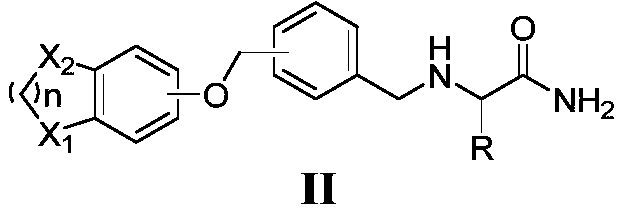
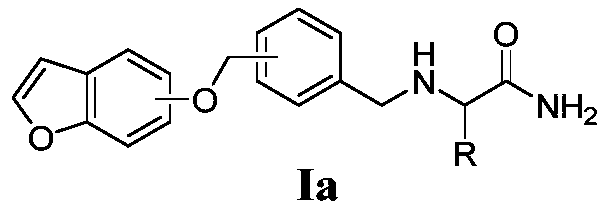
Amide derivative containing benzoheterocycle structure, and composition and application thereof
A technology of amide derivatives and heterocycles, applied in the field of medicine, can solve the problems of toxic side effects, no neuropathic pain, etc., and achieve the effect of strong analgesic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Embodiment 1 (S)-2-(4-((benzofuran-4-)oxymethyl) benzylamino) propanamide (Ia-1) synthetic
[0141]
[0142] 1.1 Synthesis of p-hydroxymethylbenzaldehyde (2a-1) and m-hydroxymethylbenzaldehyde (2a-2)
[0143]
[0144] Take a 250mL eggplant-shaped bottle, add 10.00g (74.55mmol) of terephthalaldehyde 1a-1 or isophthalaldehyde 1a-2, and then add 50mL of ethanol and 80mL of tetrahydrofuran to dissolve it. Under stirring in an ice bath, 0.85 g (22.46 mmol) of sodium borohydride was added three times. After the injection was completed, the reaction was continued for more than 6 h under ice bath conditions. Monitor the reaction process with thin-layer chromatography until point 1a-1 or 1a-2 disappears completely, stop the reaction, quench the reaction solution with 3mol / L hydrochloric acid, adjust the pH to 4–5, filter, and distill the filtrate to remove the solvent under reduced pressure, leaving The substance is a brownish yellow oil, the residue is dissolved with 50 ...
Embodiment 2
[0154] Synthesis of Example 2 (S)-2-(3-((benzofuran-4-)oxymethyl)benzylamino)propanamide (Ia-2)
[0155]
[0156] Take one 100mL eggplant-shaped bottle, add 0.59g (4.70mmol) L-alaninamide hydrochloride, 0.95g (9.40mmol) triethylamine and 30mL methanol, stir at room temperature for 1h, and dissolve 0.79g (3.13mmol) 4a -2 Add the reaction solution, continue the reaction at room temperature for 2 hours, add 1.01g (18.80mmol) potassium borohydride to the reaction solution three times, and react for 3 hours under reflux after throwing, monitor the reaction process with thin-layer chromatography until point 4a-2 completely disappears, and depressurize The solvent was distilled off, and the residue was a light yellow solid. The residue was mixed with 100-200 mesh silica gel and separated by column chromatography (gradient elution: MeOH / DCM, 0-5%) to obtain a light yellow oil (Ia-2 , 0.93g, yield 91.18%). ESIMS m / z 325.15[M+H] + ;1H NMR(400MHz,DMSO-d6):7.91(d,1H),7.21–7.48(m,7H),...
Embodiment 3
[0157] Synthesis of Example 3 (S)-2-(4-((benzofuran-5-)oxymethyl)benzylamino)propanamide (Ia-3)
[0158]
[0159] 3.1 Synthesis of 4-((benzofuran-5-)oxymethyl)benzaldehyde (4a-3) and 3-((benzofuran-5-)oxymethyl)benzaldehyde (4a-4)
[0160]
[0161] Take a 100mL eggplant-shaped bottle, add 0.69g (5.15mmol) 5-hydroxybenzofuran, 1.74g (5.35mmol) Cs 2 CO 3 and 30mL of absolute ethanol, stirred at room temperature for 1h, 1.02g (5.15mmol) 3a-1 or 3a-2 and 0.13g (0.77mmol) KI were put into the reaction solution, and the reaction was refluxed at 80°C for more than 4h after the throwing was completed. Monitor the reaction process by layer chromatography until the 5-hydroxybenzofuran point completely disappears, filter, and distill the filtrate to remove the solvent under reduced pressure. The residue is a yellow solid. Dissolve the residue with 20 mL each of water and ethyl acetate. Extract twice with ethyl acetate, 10 mL each time, combine the organic phases, wash with satura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com