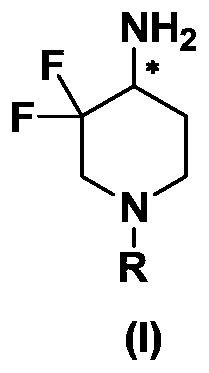
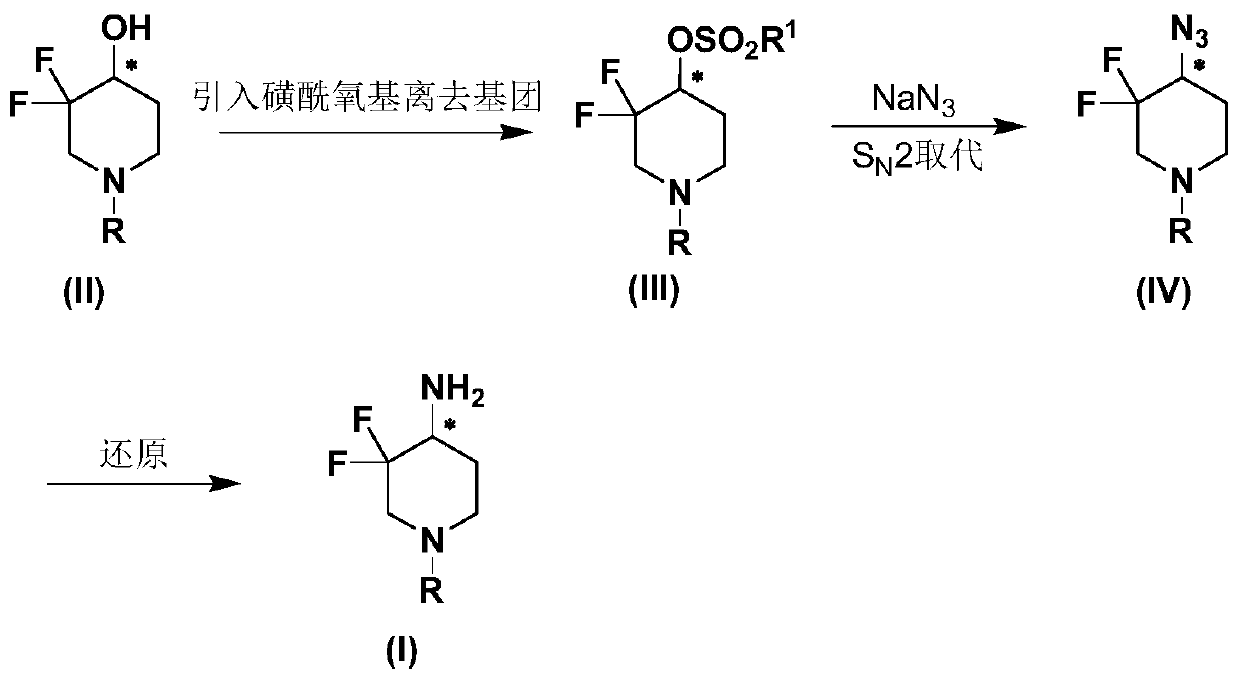
Preparation method of 3, 3-difluoro-4-aminopiperidine compound and derivative thereof
A technology of aminopiperidine and compounds, which is applied in the field of drug synthesis, can solve the problems that restrict the mass production of products, and achieve the effects of simplified operation, high yield and reasonable technical scheme
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation (S)-N-tert-butoxycarbonyl-3,3-difluoro-4-trifluoromethylsulfonyloxypiperidine (VI)
[0044]
[0045] Under nitrogen protection, the compound (S)-N-tert-butoxycarbonyl-3,3-difluoro-4-hydroxypiperidine (100g, 0.42mol, 1.00eq) represented by formula (V) was dissolved in 800mL of dichloromethane , Pyridine (100.0g, 1.26mol, 3.00eq) was added, the mixed solvent was cooled to minus 40°C, and trifluoromethanesulfonic anhydride (177.7g, 0.63mol, 1.50eq) was slowly added. The obtained light yellow clear liquid was slowly returned to room temperature, and the stirring was continued at room temperature for 2 hours, and then the reaction was monitored for completion. The reaction solution was quenched by adding 600 mL of water, and the layers were separated. The separated organic phase was washed once with saturated brine, dried over anhydrous sodium sulfate, and filtered. The solvent was evaporated under reduced pressure to obtain 155.0 g of a light y...
Embodiment 2
[0048] Embodiment 2: Preparation (S)-N-benzyl-3,3-difluoro-4-trifluoromethylsulfonyloxypiperidine (VIII)
[0049]
[0050] Under nitrogen protection, the compound (R)-N-benzyl-3,3-difluoro-4-hydroxypiperidine (100g, 0.44mol, 1.00eq) represented by the formula (VII) was dissolved in 800mL of dichloromethane, added Pyridine (104.4g, 1.32mol, 3.00eq), the mixed solvent was cooled to minus 40°C, and trifluoromethanesulfonic anhydride (186.1g, 0.66mol, 1.50eq) was added slowly. The obtained pale yellow clear liquid was slowly returned to room temperature, and the stirring was continued at room temperature for 1.5 hours, and then the reaction was monitored for completion. The reaction solution was quenched by adding 600 mL of water, and the layers were separated. The separated organic phase was washed once with saturated brine, dried over anhydrous sodium sulfate, and filtered. The solvent was evaporated under reduced pressure to obtain 159.2 g of a light yellow solid, and the c...
Embodiment 3
[0055] Embodiment 3: Preparation (R)-N-tert-butoxycarbonyl-3,3-difluoro-4-azidopiperidine (IX)
[0056]
[0057] Compound N-tert-butoxycarbonyl-3,3-difluoro-4-trifluoromethylsulfonyloxypiperidine (20g, 54.2mmol, 1.00eq) represented by formula (VI) was dissolved in anhydrous DMF (150mL) In, add sodium azide (5.28g, 81.2mmol, 1.5eq) and stir well. The resulting mixture was warmed to 40°C and stirred for 12 hours. After detecting the completion of the reaction, the reaction was lowered to room temperature, and 200 mL of saturated sodium chloride and 200 mL of ethyl acetate were added to separate the liquid. The aqueous phase was extracted once with ethyl acetate, and the organic phase was combined and washed with saturated brine (200 mL X2). Anhydrous sodium sulfate After drying, filtration and concentration, the crude product was obtained which could be directly used in the next reaction (light yellow oil, 12.78 g, 48.8 mmol, purity 95%, yield 90%).
[0058] Compound shown ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com