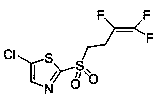
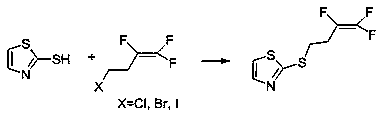
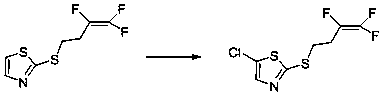
Method for synthesizing fluensulfone
A technology of fluthiandin and thiazole, which is applied in the field of synthesis and preparation of pesticide raw materials, can solve the problems of environmental pollution, many solid wastes and the like, and achieves the effects of high yield, short reaction steps and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Synthesis of 2-(3,3,4-trifluoro-3-butenylmercapto)thiazole
[0026] Under nitrogen protection, 26g mercaptothiazole, 33.6g sodium carbonate were used as catalyst and acid binding agent and 46g 4-chloro-1,1,2-trifluoro-1-butene were reacted, heated in 300ml dimethylformamide After reflux for 6-8 hours, after cooling to room temperature, filter the mixture, evaporate the solvent, dissolve the residue with 200ml of dichloromethane, wash with 200ml of 5% aqueous sodium hydroxide solution and 200ml of water successively, and then dry with anhydrous sodium sulfate. Filter and spin dry to obtain 40 g of 2-(3,3,4-trifluoro-3-butenylmercapto)thiazole with a yield of 80%.
Embodiment 2
[0027] Example 2 Synthesis of 2-(3,3,4-trifluoro-3-butenylmercapto)-5-chlorothiazole
[0028] 40g of 2-(3,3,4-trifluoro-3-butenylmercapto)thiazole was dissolved in 350ml of dichloromethane, 28.5g of dichlorohydantoin was added to the solution, and heated to reflux for 18 hours under nitrogen protection . After cooling to room temperature, the solid was filtered out, the reaction solution was washed with water, and the solvent was distilled off to obtain 45 g of 2-(3,3,4-trifluoro-3-butenylmercapto)-5-chlorothiazole. The yield is 98%.
Embodiment 3
[0029] Example 3 Synthesis of fluthiazol
[0030] Add 45g of 2-(3,3,4-trifluoro-3-butenylmercapto)-5-chlorothiazole to 45ml of water, add 1.74g of catalyst sodium phosphotungstate, and then within 30min at 5°C, While stirring, 67 g of hydrogen peroxide was added dropwise. The temperature was raised to 65°C and stirred for 1 hour. The peroxide test was performed by adding 18 g of sodium bisulfite to remove excess hydrogen peroxide. Filter and spin off the methanol component. Extracted with 50ml of ethyl acetate, dried over sodium sulfate, and spin-dried to obtain 43g of yellow oil with a yield of 86%. 1 HNMR (500 MHz, CDCl 3 ), δ: 7.89 (s, 1H), 3.64 (m, 2H), 2.93 (d, J = 19.6, 7.0, 3.2 Hz, 2H). 13 CNMR (125MHz, CDCl 3 ), δ: 19.90, 20.10, 29.33, 50.38, 135.41, 143.35, 162.51.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com