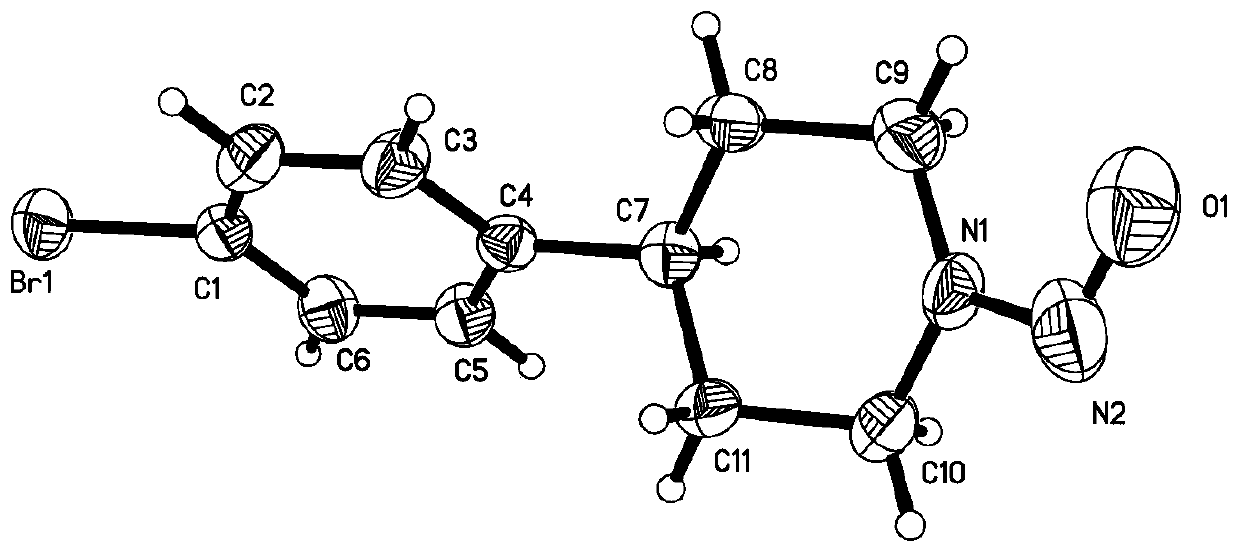
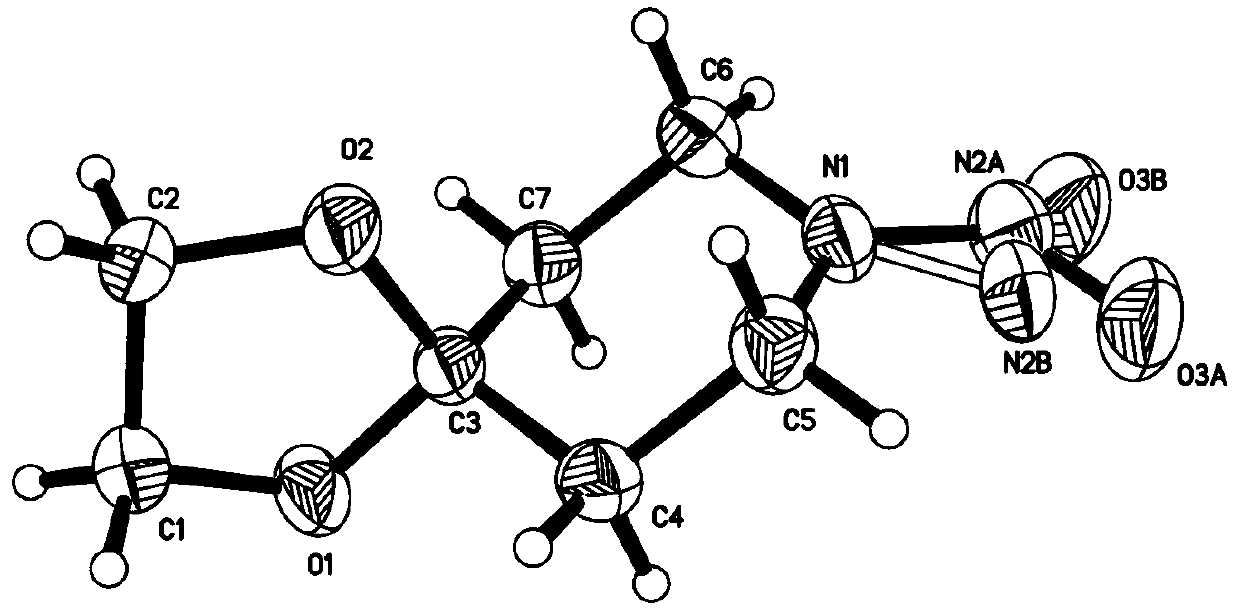
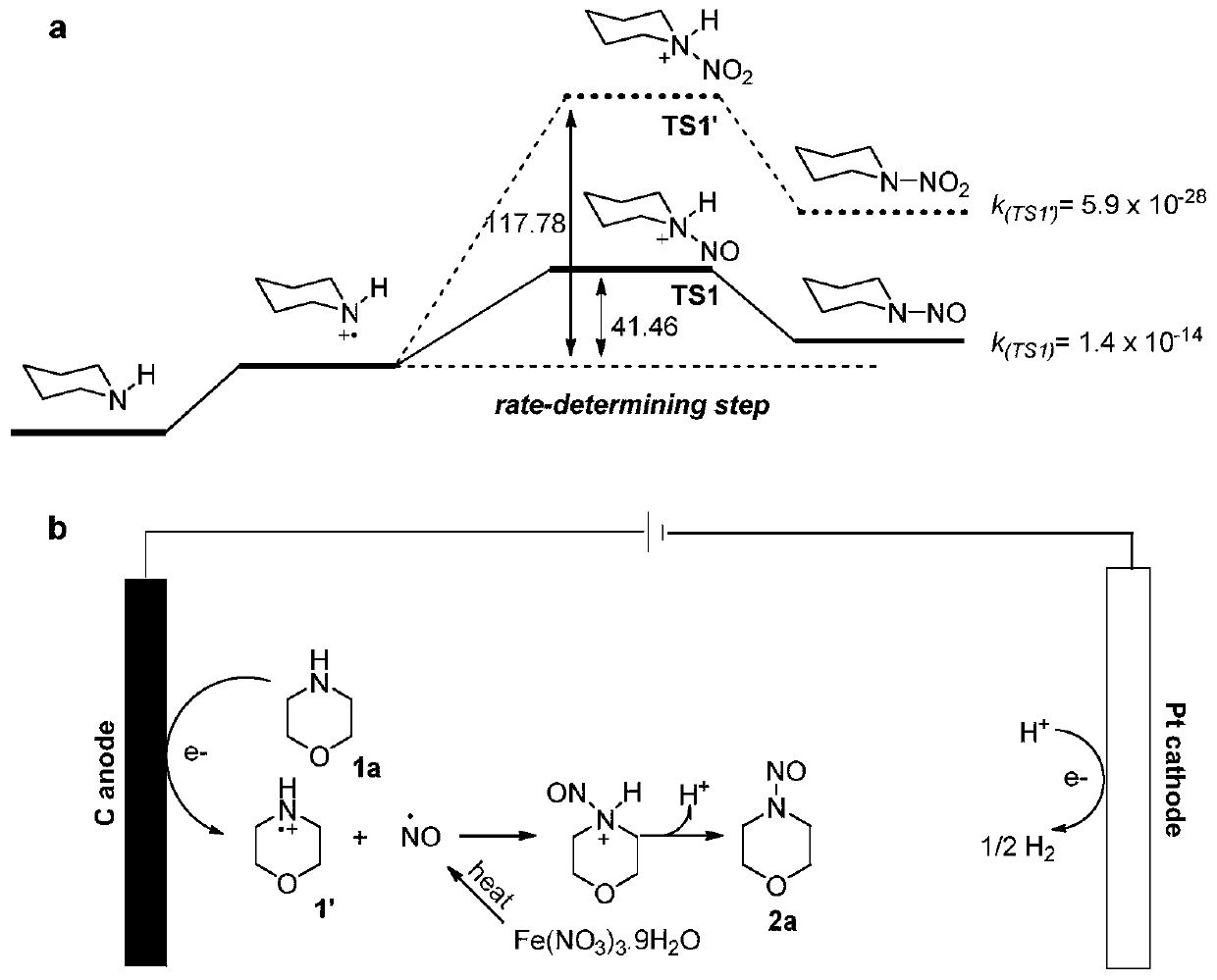
Synthesis method of nitrogen nitrosation product of secondary amine
A synthesis method and nitrosation technology, applied in electrolytic organic production, electrolytic components, electrolytic process and other directions, can solve the problems of side reactions, harsh reaction conditions, long reaction time, etc., and achieve high yield and good substrate universality. , the effect of simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of N-nitrosomorpholine
[0029]
[0030] In an oven-dried three-neck flask (25mL) equipped with a stirring bar, add morpholine (1.0mmol, 87mg), electrolyte n-Bu 4 NBF 4 (205mg, 0.6mmol) and ferric nitrate nonahydrate (2.0mmol, 0.808g). The three-necked flask is equipped with a graphite rod (Φ=6mm) as the anode, and a platinum plate electrode (10mm×10mm) as the cathode, and 16mL of CH 3 CN, then evacuated and flushed with nitrogen as a protective gas. The reaction mixture was stirred at reflux for 4 hours at a constant current of 12 mA at 70°C. When the reaction was completed, the reaction mixture was washed with water twice and extracted with ethyl acetate (10 mL×3). Then the organic layers were combined and washed with Na 2 SO 4 Concentrate in vacuo after drying, then obtain pure product by flash chromatography column, be light yellow oily liquid, yield rate is 92%, the NMR characteristic of this product is: 1 H NMR (500MHz, DMSO-d 6 )δ=4.26–4.16(m...
Embodiment 2
[0032] Synthesis of N-nitrosomorpholine
[0033]
[0034] The reaction steps are exactly the same as in Example 1, except that ferric nitrate is changed into bismuth nitrate, and the product is light yellow oily liquid with a yield of 62%. The NMR of this product is characterized as: 1 H NMR (500MHz, DMSO-d 6 )δ=4.26–4.16(m,2H),3.85–3.77(m,2H),3.77–3.73(m,2H),3.58–3.54(m,2H). 13 C NMR (126MHz, DMSO-d 6 ) δ=66.67, 65.18, 49.59, 40.30.
Embodiment 3
[0036] Synthesis of N-nitrosopiperidine
[0037]
[0038] The reaction steps are exactly the same as in Example 1, except that morpholine is changed to piperidine, and the reaction time is extended to 5h. The product is a light yellow oily liquid with a yield of 88%. The NMR of this product is characterized as: 1 H NMR (500MHz, Chloroform-d) δ=4.21–4.16(m,2H), 3.81–3.76(m,2H), 1.83–1.74(m,4H), 1.56(p,J=5.9,2H). 13 C NMR (126 MHz, Chloroform-d) δ = 51.04, 39.99, 26.59, 24.92, 24.32.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com