Albumin nanoparticle composition and its preparation method
A technology of albumin nanoparticles and composition, applied in the field of medicinal chemistry, can solve the problems affecting the clinical drug benefit/risk ratio of gemcitabine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
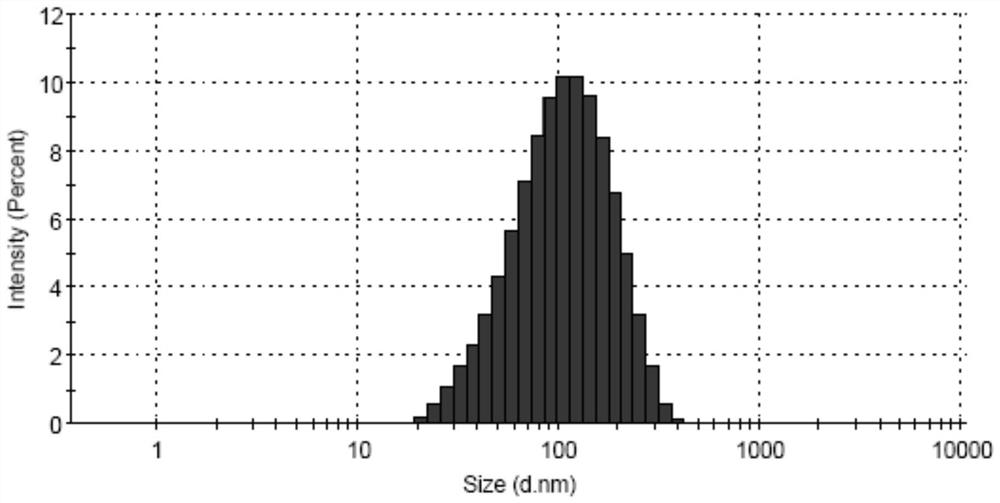
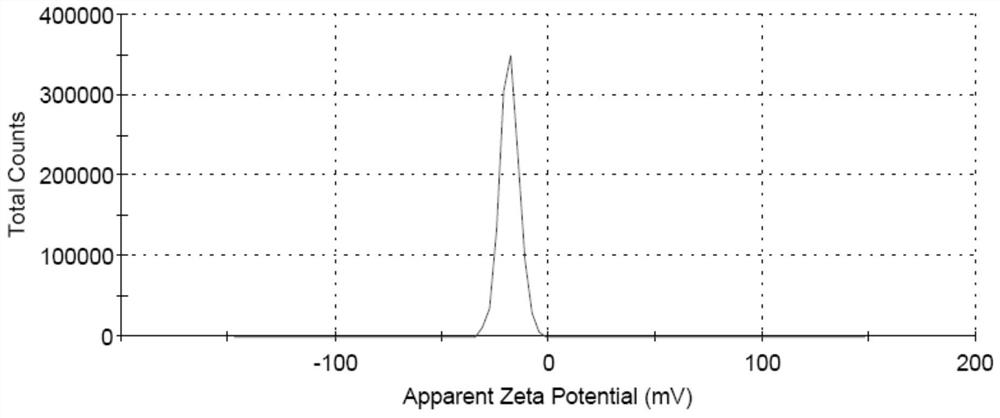
Image
Examples
Embodiment 1
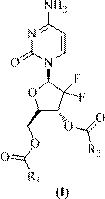
[0175] Example 1. Preparation of 2', 2'-difluoro-2'-deoxycytidine-3', 5'-di-n-decanoate
[0176] The schematic reaction scheme is as follows:
[0177]
[0178] Dissolve commercially available 2',2'-difluoro-2'-deoxycytidine-3',5'-dibenzoate (compound of formula II, 4.7 g, 0.01 mol) in 50 mL of dioxane ring, then add triethylamine (4.0g, 0.04mol), N,N-lutidine (1.8g, 0.01mol) and Boc 2 O (12.9 g, 0.04 mol). The reaction was carried out at 40° C. for 5 hours, and the reaction was monitored by TLC to complete. Post-treatment: Pour the reaction liquid into water, extract with dichloromethane, concentrate the organic phase (to obtain compound III) and proceed to the next reaction directly. The above compound III was dissolved in 20 mL of methanol solution, then solid NaOH (28 mg, 0.7 mmol) was added, stirred at room temperature for 4 hours, and the reaction was monitored by TLC. Post-treatment: Filtrate directly with silica gel, concentrate the filtrate and perform column ...
Embodiment 2
[0183] Example 2, preparation of 2',2'-difluoro-2'-deoxycytidine-3',5'-dilaurate
[0184] The preparation method refers to Example 1. Compound IV is reacted with lauroyl chloride, and the Boc protecting group is removed by trifluoroacetic acid to obtain a white solid as the title compound.
[0185] 1 H NMR (500MHz, CDCl 3 )δ7.55(brs,1H),6.32(brs,1H),6.09(brs,1H),5.29(brs,1H),4.34-4.45(m,3H),2.37-2.40(m,4H),1.60 -1.70(m,4H),1.25-1.29(m,32H),0.92(t,J=6.50Hz,6H).MS-ESI(m / z):628.3(M+H) + .
Embodiment 3
[0186] Example 3, Preparation of 2',2'-difluoro-2'-deoxycytidine-3',5'-dimyristate
[0187] The preparation method refers to Example 1. Compound IV is reacted with myristoyl chloride, and the Boc protecting group is removed by trifluoroacetic acid to obtain a white solid as the title compound.
[0188] Mp:142-144℃; 1 H NMR (500MHz, CDCl 3 )δ7.44(d, J=6.85Hz, 1H), 6.44(d, J=8.85Hz, 1H), 5.74(d, J=7.30Hz, 1H), 5.22(d, J=11,90Hz, 1H ),4.38(brs,2H),4.26(brs,1H),2.41(t,J=7.35Hz,2H),2.36(t,J=7.35Hz,2H),1.60-1.70(m,4H),1.25 -1.29(m,40H),0.87(t,J=6.50Hz,6H).MS-ESI(m / z):684.4(M+H) + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com