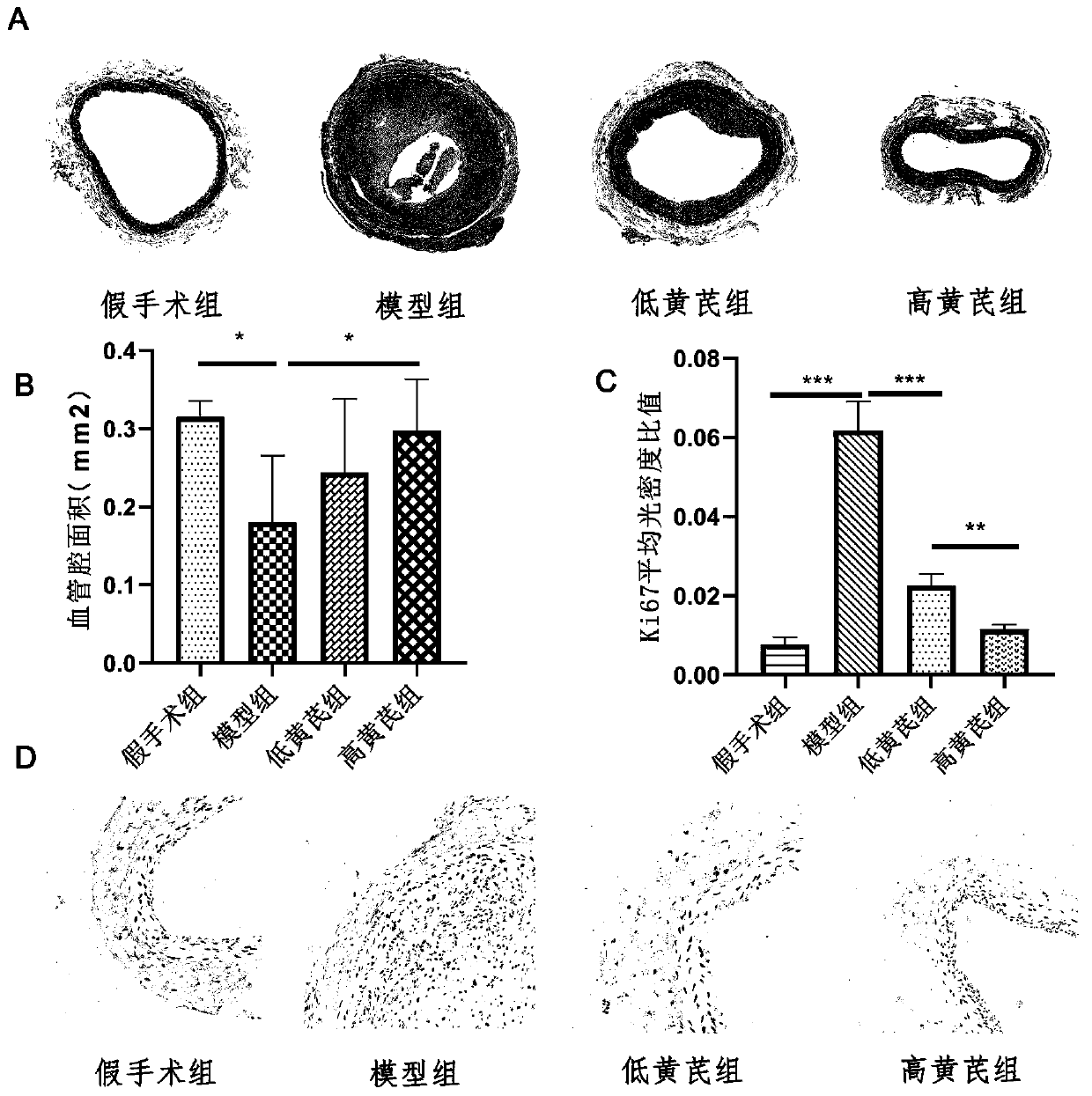
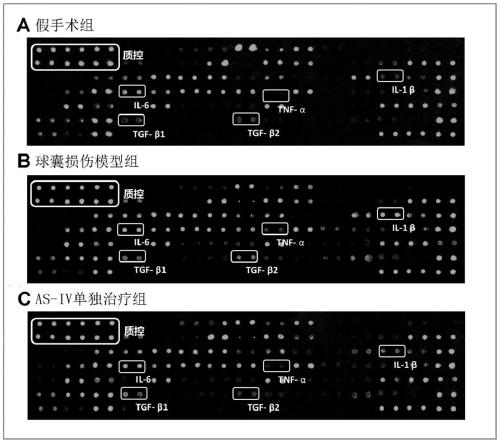
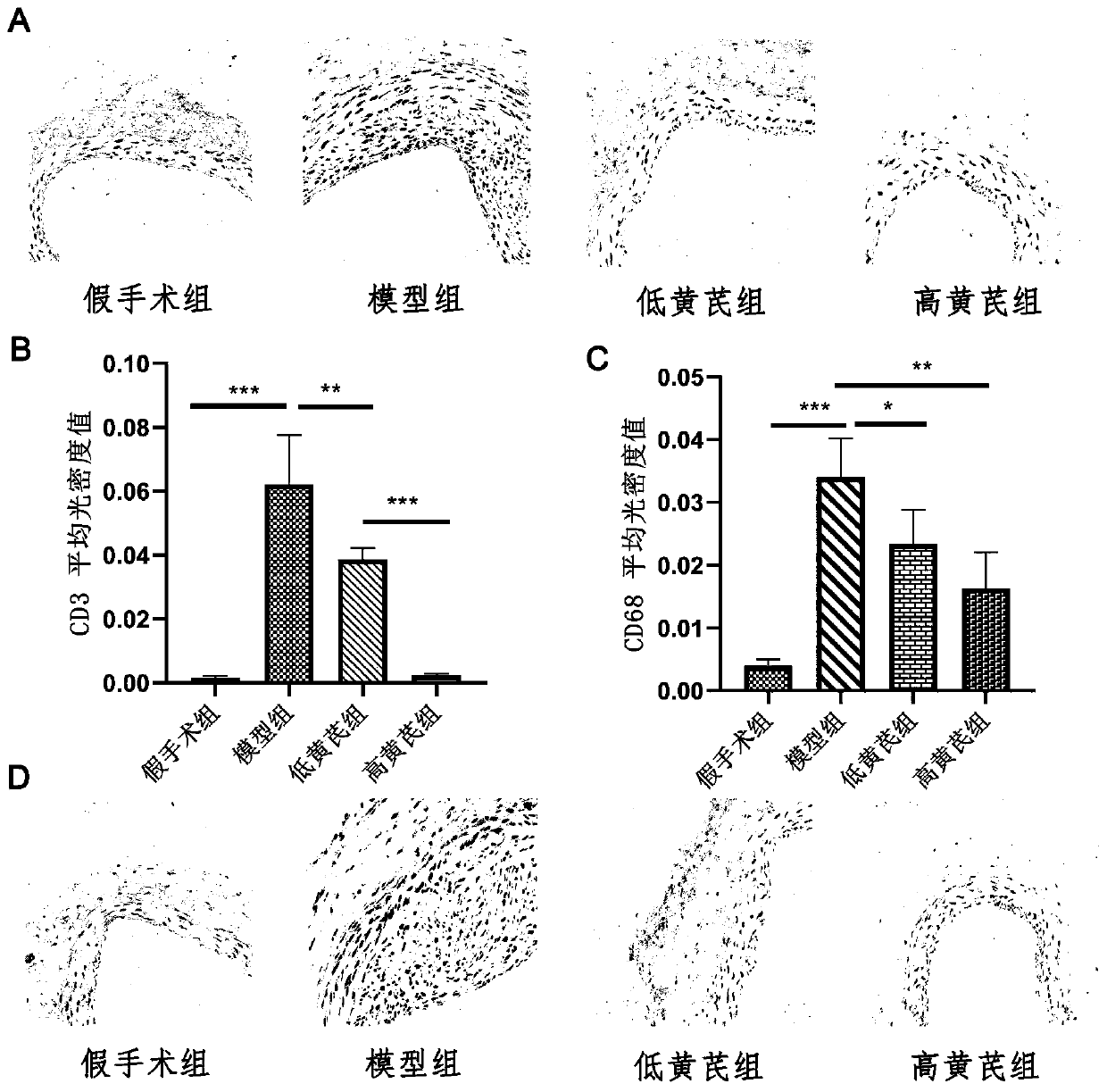
Application of astragaloside IV in inhibiting local inflammatory response and treating arterial restenosis
An astragaloside IV, inflammatory reaction technology, applied in the directions of anti-inflammatory agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problem that the mechanism of occurrence is not fully understood and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Main instruments and consumables:
[0029]-80°C ultra-low temperature refrigerator, liquid nitrogen, autoclave, Eppendorf micropipette, Image Quant LAS500 imager, microplate reader, electrophoresis apparatus, vortex mixer, high-speed cold centrifuge, ultra-clean bench, electronic balance , balloon catheter
[0030] 2. Main reagents:
[0031] AS-IV (Beijing Apprise Technology Company), CD3 immunohistochemistry kit, CD68 immunohistochemistry kit, Ki67 immunohistochemistry kit, chloral hydrate, sodium carboxymethylcellulose (Beijing Dingguo Biotechnology Co., Ltd. company), PCNA, a-SMA (Abcam Biological Company of the United States), ICAM-1, β-actin, goat anti-rabbit secondary antibody, BSA (Wuhan Aibotek Biological Company).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com