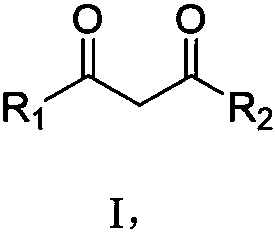
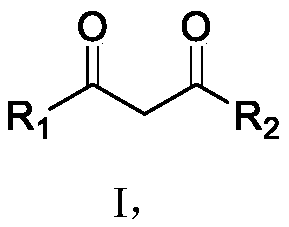
Synthesis method of polycyclic disubstituted 1,3-propanedione compound
A synthesis method and compound technology, applied in the field of organic compound synthesis, can solve the problems of high operating level requirements, unsuitable for industrial production, easy to generate hydrogen, etc., and achieve the effects of easy operation, not easy to flash explosion, and promoting the reaction to occur.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
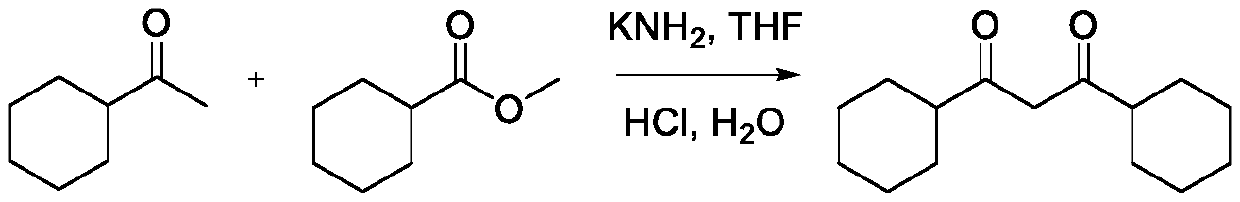
[0030] Synthesis of 1,3-dicyclohexyl-1,3-propanedione
[0031]
[0032] Under the condition of nitrogen protection, 1-cyclohexyl-1-ethanone (18.9g, 150mmol,) and methyl cyclohexanecarboxylate (14.2g, 100mmol) were dissolved in 80mL of anhydrous tetrahydrofuran, and potassium amide (11.0g , 200mmol), stirred at room temperature (23°C) for 3.5h. After the reaction, the reaction solution was poured into ice water, and diethyl ether was added to separate the layers. The aqueous layer was first adjusted to pH=5.0 with 2N HCl, and then Na 2 CO 3 Adjust to pH=7.5, extract with ether, and dry over anhydrous magnesium sulfate. Most of the solvent was spun off, purified by silica gel column chromatography, and eluted with dichloromethane / methanol (V / V=20 / 1) to obtain 1,3-dicyclohexyl-1,3-propanedione (19.9g ). Yield: 84%.
[0033] NMR test results:
[0034] 1 H NMR (400MHz, CDCl 3 )δ15.75(br,1H),5.49(s,1H),2.21-2.14(m,2H),1.88-1.79(m,8H),1.73-1.68(m,2H),1.41-1.17(m, 10H).
Embodiment 2
[0036] Synthesis of 1,3-dicyclopentyl-1,3-propanedione
[0037]
[0038] The raw materials used in this example are 1-cyclopentyl-1-ethanone and methyl cyclopentacarboxylate, the molar ratio of 1-cyclopentyl-1-ethanone and methyl cyclopentacarboxylate is 1.5:1, other As in Example 1, 1,3-dicyclopentyl-1,3-propanedione was finally obtained with a yield of 85%.
[0039] NMR test results:
[0040] 1 H NMR (400MHz, CDCl 3 )δ15.66(br,1H),5.56(m,1H),2.59(m,1H),2.44(m,1H),1.79-1.76(m,3H),1.74(m,1H),1.70-1.65 (m,10H).
[0041] 13 C NMR (125MHz, CDCl 3 )δ205.00(d), 54.62(dt), 51.13(dtd), 28.91(dt), 25.50(td).
Embodiment 3
[0043] Synthesis of 1,3-dicyclobutyl-1,3-propanedione
[0044]
[0045] The raw materials used in this example are 1-cyclobutyl-1-ethanone and methyl cyclobutanecarboxylate, the molar ratio of 1-cyclobutyl-1-ethanone and methyl cyclobutanecarboxylate is 1.5:1, other As in Example 1, 1,3-dicyclobutyl-1,3-propanedione was finally obtained with a yield of 85%.
[0046] NMR test results:
[0047] 1 H NMR (400MHz, CDCl 3 )δ5.56(d,1H),2.67(m,1H),2.47(m,1H),1.99-1.94(m,8H),1.76-1.73(m,4H).
[0048] 13 C NMR (125MHz, CDCl 3 )δ205.08(d), 55.10(dt), 47.45(dh), 28.07(t), 20.56(dt).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com