Catalyst for preparing saturated lubricant base oil
A technology of diphenylmethyl and trifluoromethylaniline, which is applied in the field of catalysts for the preparation of saturated lubricating oil base oils, can solve problems such as complex processes, easy-to-corrosion equipment, and harsh reaction conditions, and achieve enhanced electrophilicity and improved catalytic performance. The effect of activity and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment provides a kind of 2,6-bis(benzhydryl)-4-trifluoromethylaniline, and its synthesis method is as follows.
[0032] Synthesis of 2,6-bis(benzhydryl)-4-trifluoromethylaniline: 4-trifluoromethylaniline (3.78g, 23.5mmol) and benzhydryl alcohol (8.65 g, 47mmol), equipped with a reflux device, heated to (about 70 ° C), after p-toluidine and benzhydryl melted, dissolved ZnCl with concentrated HCl 2 (3g, 23.5mmol), dropwise added ZnCl 2 ·The HCl mixed solution was heated up to 160°C and reacted for 3 hours. After the reaction stopped, cool to room temperature and use CH 2 Cl 2 Dissolve solids, extract, retain organic phase, add Na 2 CO 3 Remove excess HCl, filter, add silica gel to the filtrate to absorb impurities, filter, spin the filtrate to obtain a solid powder, wash with ethyl acetate to obtain a white powder, and the yield is 78.0%. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):7.30-7.16(m,20H,Ph),6.90(s,2H,Ar-H),5.58(s,2H,CH),5.32(s,2H,NH 2 ). 13 C-NMR (100MH...
Embodiment 2
[0034] This embodiment provides a pyridine-imine ligand, and its synthesis method is as follows.
[0035] Synthesis of pyridine-imine ligand: Weigh 2-acetylpyridine (0.605g, 5.0mmol), 2,6-bis(benzhydryl)-4-trifluoromethylaniline (2.98g, 5.0mmol) 1. An appropriate amount of p-toluenesulfonic acid was dissolved in 100 mL of toluene, refluxed for 10 h, and the crude product after the solvent was spin-dried was recrystallized with ethanol to obtain yellow crystals with a yield of 85.2%. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):8.80(d,1H,Py),8.52(d,1H,Py),7.91(t,1H,Py),7.64(t,1H,Py),7.30-7.10(m,12H ,Ph),7.08(t,8H,Ph),6.87(s,2H,Ar-H),6.68(s,2H,CH),5.52(s,1H,CH),2.02(s,3H,CH 3 ). 13 C-NMR (100MHz, CDCl 3 ), δ(ppm): 170.50, 165.37, 155.04, 150.24, 145.45, 144.25, 139.63, 138.26, 130.68, 129.76, 126.68, 123.56, 116.58, 52.15, 17.88.
Embodiment 3
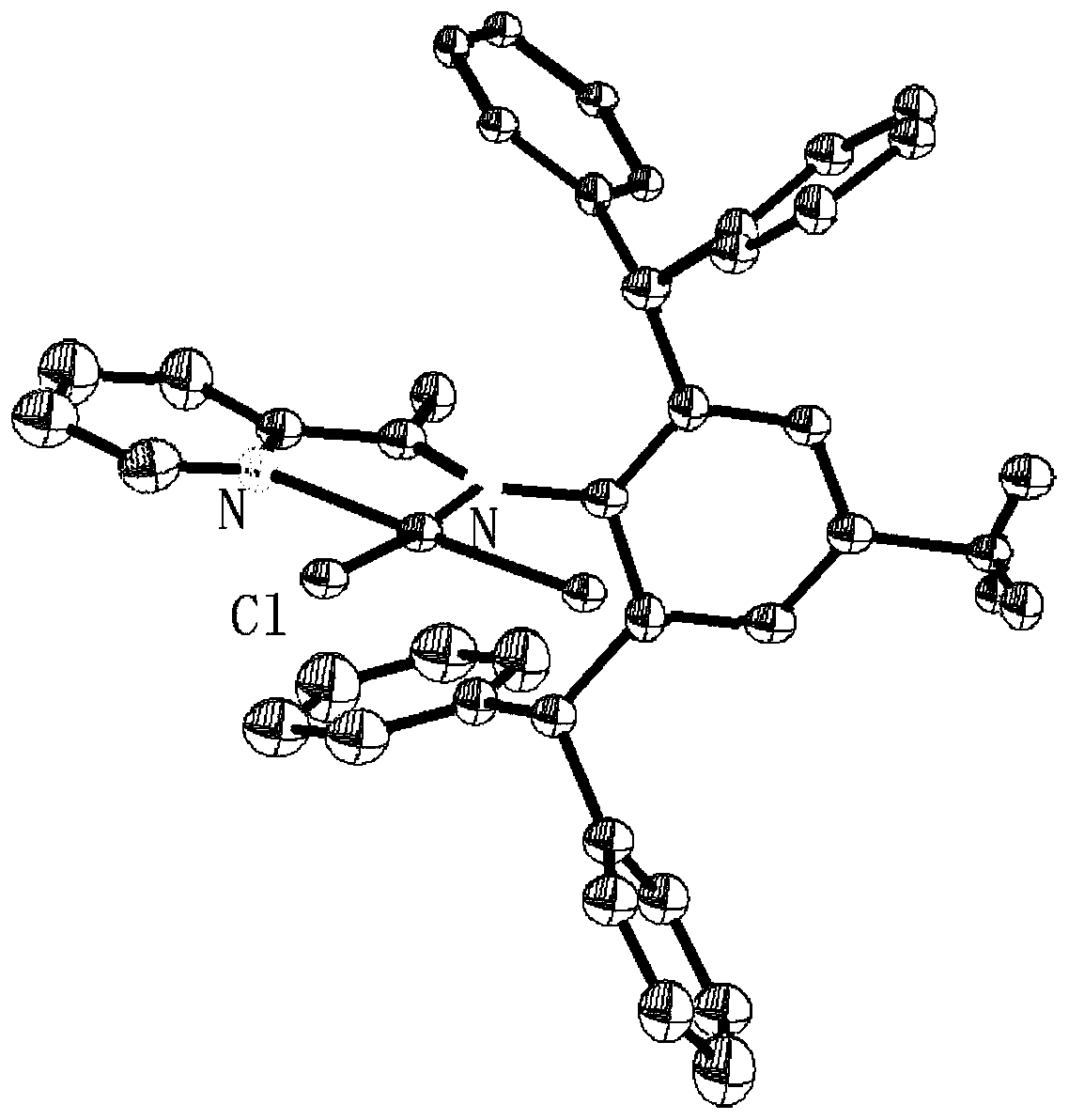
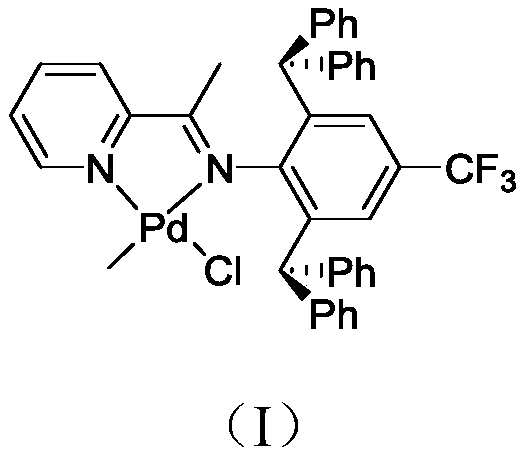
[0037] This example provides a pyridine-imine palladium complex, the synthesis method of which is as follows.
[0038] Add 1.2 mmol of pyridine-imine ligand, 1.0 mmol of (COD)PdMeCl, and 20 mL of dichloromethane into a Schlenk test tube, and stir overnight at room temperature. The solution was evaporated to 5 mL under vacuum, then 30 mL of n-hexane was added. The yellow powder pyridine-imine palladium complex was obtained by filtration with G4 filter ball, and the yield was 82.5%. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):8.60(d,1H,Py),8.05(d,1H,Py),7.73(t,1H,Py),7.34(t,1H,Py),7.28-7.10(m,12H ,Ph),7.02(t,8H,Ph),6.69(s,2H,Ar-H),5.26(s,2H,CH),2.19(s,3H,CH 3 ),1.06(s,3H,Pd-CH 3 ). 13 C-NMR (100MHz, CDCl 3), δ(ppm): 169.71, 156.25, 148.56, 146.42, 144.15, 142.76, 136.42, 132.50, 131.52, 129.65, 128.78, 126.52, 121.36, 52.42, 21.57, 17.20.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecule | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com