A kind of self-healing polyurethane elastomer without external stimulation and preparation method thereof
A technology of polyurethane elastomer and external stimulation, which is applied in the field of polymer materials, can solve problems such as no relevant literature reports, and achieve the effects of improving application prospects, simple synthesis process, and good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034]The preparation method of the dihydric alcohol chain extender containing an acylhydrazone bond comprises the following steps: dissolving 12-30 parts of dihydrazide compound in deionized water or an organic solvent, dissolving 15-42 parts of α- The hydroxycarbonyl compound is dissolved in deionized water or an organic solvent, mixed, and reacted at a temperature of 20°C-60°C for 2-4h to obtain a hydroxyl-terminated chain extender containing an acylhydrazone bond. The organic solvent is dimethyl sulfoxide, isopropanol, ethyl acetate, N, N-dimethylformamide, glacial acetic acid or acetone; the mixed weight part of the deionized water and / or organic solvent is 130 - 140 parts by weight.
[0035] The dihydrazide compound is adipic dihydrazide, butenedihydrazide, succinic acid dihydrazide, adipic acid dihydrazide, 1,3-benzoic acid dihydrazide, phthalic acid dihydrazide, terephthalic acid dihydrazide, Phthalic acid dihydrazide, sebacic acid dihydrazide, pyridine-2,6-dicarboxyl...
Embodiment 11
[0041] Example 1.1: Preparation of a dihydric alcohol chain extender containing an acylhydrazone bond
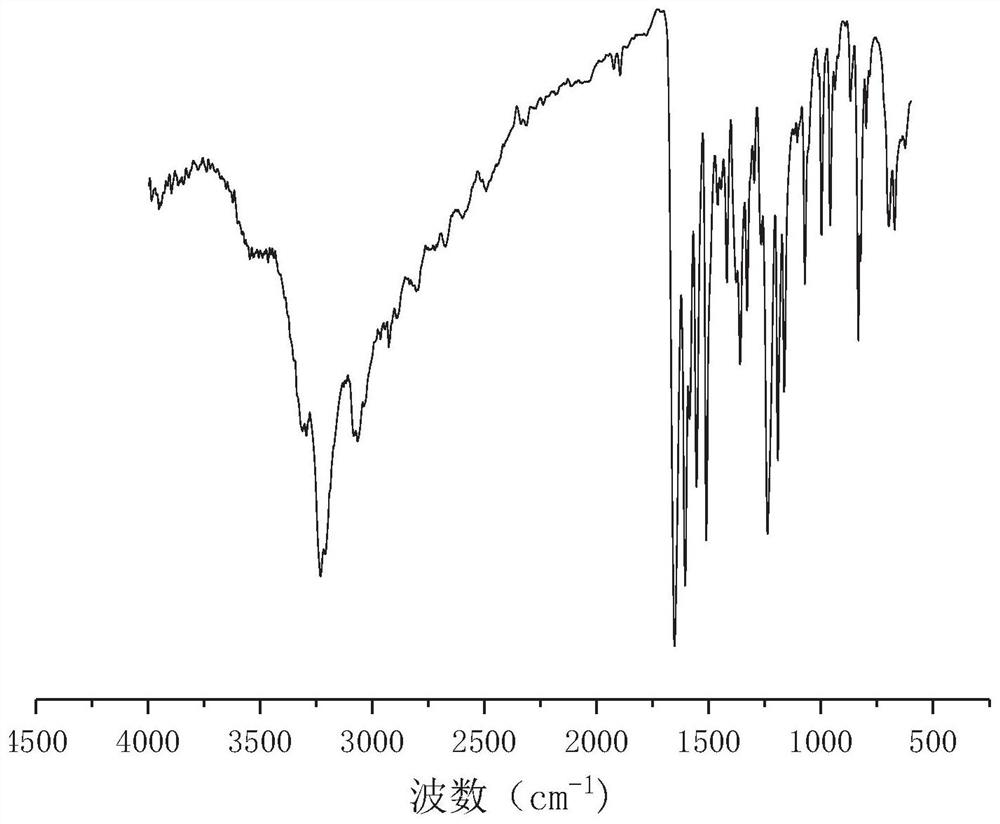
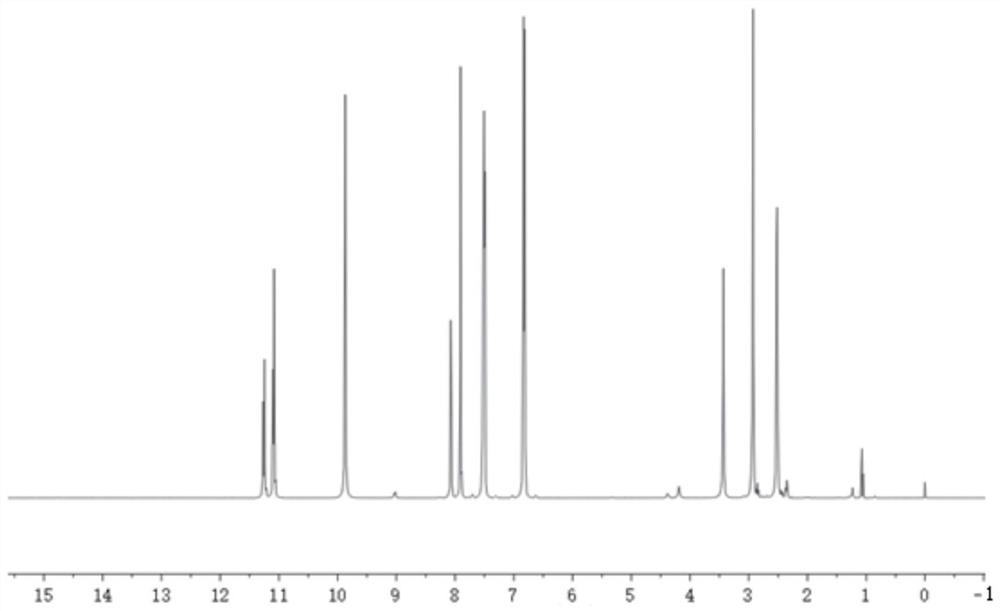
[0042] Get p-Hydroxybenzaldehyde 24.4g (0.2mol-CHO) and dissolve it in 80g absolute ethanol, get succinic acid dihydrazide 14.6g (0.2mol hydrazide group) and dissolve it in 80.0g deionized water; After mixing evenly, react at 25°C for 2.5 hours under stirring at 250 rpm. Filtration under reduced pressure, the resulting product is washed with a 1:1 mixed solution of absolute ethanol and deionized water, and dried to obtain a dihydric alcohol chain extender containing an acylhydrazone bond. The molecular structural formula of the obtained product is: Named as p-hydroxybenzaldehyde succinate dihydrazone, the infrared spectrum of p-hydroxybenzaldehyde succinate dihydrazone is shown in figure 1 , see the H NMR spectrum figure 2 . The product is a white solid powder with a yield of 89%.
Embodiment 12
[0043] Example 1.2: Preparation of a diol chain extender containing an acylhydrazone bond
[0044] Dissolve 13.6g (0.1mol-CHO) of 2-hydroxyacetophenone in 50g of acetone, and dissolve 9.4g of acetylsuccinic acid dihydrazide (0.1mol hydrazide group) in 40.0g of glacial acetic acid; mix the two The solution was mixed evenly and reacted at 60° C. for 4 h under stirring at 250 rpm. Filtrate under reduced pressure, and the resulting product is washed with a 1:1 mixed solution of acetone and glacial acetic acid, and dried to obtain a dihydric alcohol containing an acylhydrazone bond. The molecular structural formula of the obtained product is: Named as 2-hydroxyacetophenone succinate dihydrazone. The product is a white solid powder with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com